Deviation Management
Capture. Investigate. Prevent.
Stop accepting 'human error' as a root cause. AI-powered pattern detection finds the systemic issues behind individual deviations.
"Human error" isn't a root cause. A deviation log full of "operator error" and "retrain operator" tells regulators you don't understand your processes. The question isn't who made the mistake—it's what made the mistake possible.
AI finds what humans miss
You log a deviation: "pH excursion during hold step, Batch 2847." Before you finish typing, AI searches your deviation history and surfaces: "12 similar deviations in the past 18 months. 8 involved the same hold tank. 6 occurred during shift change. Common root cause: calibration drift on pH probe PT-401."
You were about to investigate this as an isolated event. AI showed you it's the thirteenth instance of a systemic problem. The investigation shifts from "what happened to this batch" to "why does this keep happening."
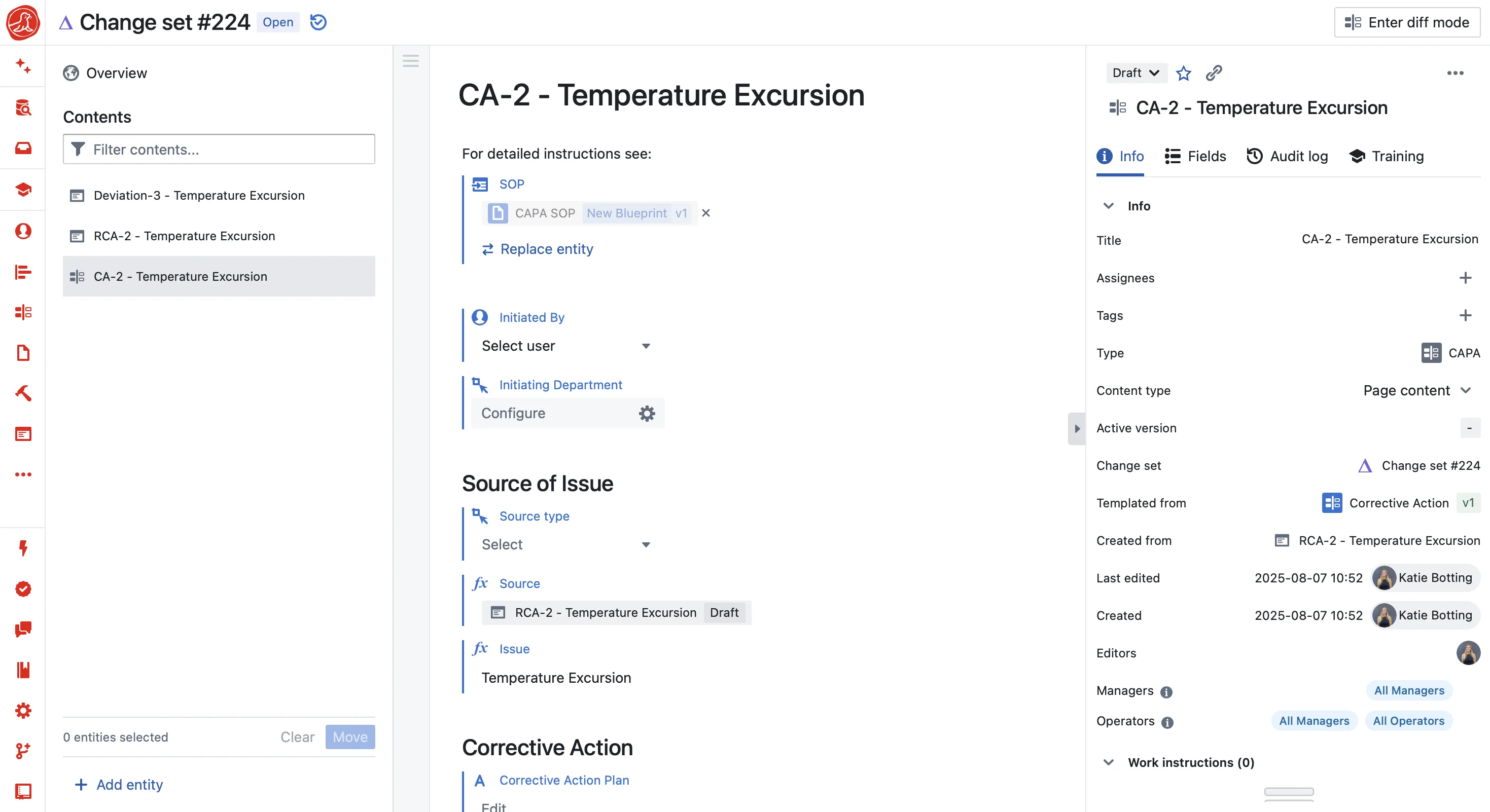
Context captured automatically
A deviation in Seal isn't a form—it's a snapshot of everything happening at that moment. Batch ID, equipment status, operator training records, process values, environmental conditions. When an auditor asks "what happened?", you show them the complete picture. When AI analyzes the deviation, it has the same context.
Equipment sensors detect out-of-spec conditions and create deviations automatically: temperature excursion duration and range, pressure spike timestamp, alarm trigger conditions. The system captures objective data. The operator adds interpretation.
Investigation with AI assistance
You start a 5-Why. AI suggests the next question based on your deviation history and industry patterns.
Why did the operator skip the step? AI suggests: "In 73% of similar deviations, procedure clarity was cited. The current SOP was revised 6 months ago." You investigate and confirm—the procedure was confusing. Why was it confusing? It was updated without usability review. Why wasn't it reviewed? No review process exists. Now you have something systemic to fix.
AI drafts the investigation summary: "Root cause: Procedure SOP-2847 section 4.3 lacks clear hold time specification, leading to operator interpretation variability. This is the fourth deviation linked to this procedure revision. Recommended action: Revise SOP with explicit hold parameters and operator verification step." You review, edit, approve.
Pattern detection across your system
Individual deviations are noise. Patterns are signal. Seal's AI continuously analyzes:
- Equipment patterns: Which assets generate the most deviations? Is Bioreactor 3 trending worse than Bioreactor 4?
- Procedural patterns: Which SOPs cause confusion? Which steps get skipped?
- Temporal patterns: Do deviations cluster by shift, day of week, time since last maintenance?
- Personnel patterns: Not to blame individuals, but to identify training gaps
When a pattern emerges, the system alerts before you've accumulated enough deviations to notice manually. The signal that took 12 incidents to see? AI flags it at 4.
Classification and escalation
Critical deviations notify QA leadership immediately and require VP approval to close. Minor documentation gaps close with QA specialist review. AI suggests classification based on deviation details and historical outcomes—you confirm or override.
Timelines are enforced. Aging deviations escalate automatically. Nothing sits in a queue waiting for someone to remember it exists.
AI that builds your deviation system
Traditional deviation management setup: months defining categories, configuring workflows, creating investigation templates. AI changes this completely.
Describe what you need: "We manufacture sterile injectables and need to track process deviations, equipment failures, and environmental excursions with different investigation depths." AI generates the configuration—deviation categories appropriate for your operation, classification criteria, escalation rules, investigation templates. You review, refine, approve through standard change control.
Investigation templates build conversationally. "Create a 5-Why template for aseptic processing deviations that captures contamination source, personnel involvement, and environmental factors." AI generates the template with appropriate fields and prompts. Your team reviews and approves.
Every AI proposal is transparent. When AI suggests a category structure or investigation workflow, you see exactly what it created. Configuration changes go through your review process. AI accelerates setup; your quality team controls the system.
And AI works within every investigation. Open a deviation—AI surfaces similar events and suggests root causes. Write an investigation summary—AI drafts from your structured findings. Close the investigation—AI flags if the root cause pattern suggests a CAPA is warranted. The same AI that configured your deviation system now accelerates every investigation within it.
Capabilities


