Manufacturing Execution System
Execute. Record. Release.
Digital batch records that enforce compliance and accelerate release.
Paper batch records are holding you back.
Every deviation, every data integrity finding, every product recall traces back to the same root cause: humans copying numbers from one piece of paper to another. The paper batch record was a reasonable solution in 1970. Today, it's a liability consuming 20-30% of manufacturing time and causing error rates as high as 25%.
You know the cost. Months of release delays while QA reviews page after page. Millions in investigations when someone transposes a digit. Regulatory observations that question your data integrity. Yet most organizations cling to paper because they've been burned by failed digital transformations—companies who bought an "Electronic Batch Record" system that promised to eliminate paper and got a PDF on a screen instead. Same rigid workflows, same manual transcription, same review process, just on an iPad instead of a clipboard.
The fundamental problem is that these systems digitized the wrong thing. They digitized the paper form instead of the manufacturing process. The form is just a documentation artifact. The process is what matters.
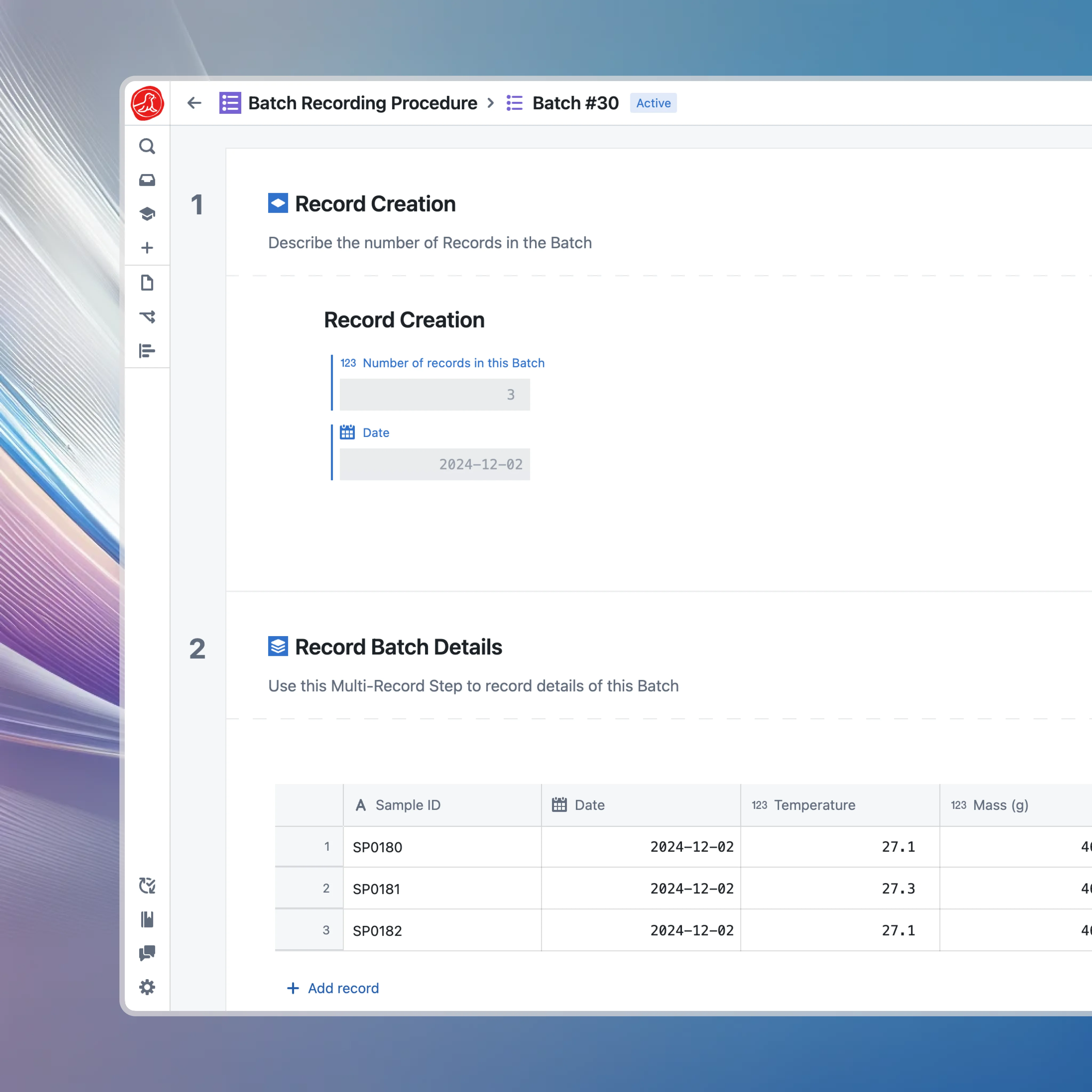
Process as data, compliance by enforcement
Seal treats your manufacturing process as structured data. A batch record isn't a form to fill out—it's a workflow to execute. Each step knows what inputs it needs, what outputs it produces, what equipment it uses, and what parameters must be within range. When an operator executes a step, they're not filling in a blank field—they're interacting with the process itself. The system knows what should happen and guides them through it. Parameters are captured from connected equipment, not typed in. Material lot numbers are scanned, not written. Calculations are performed automatically, not done on calculators and transcribed.
This structure enables something paper can never do: enforcement. An operator cannot proceed if a value is out of specification. They cannot use expired materials. They cannot skip a required verification. The compliance controls are built into the process, not enforced through review after the fact.
When compliance is enforced at the point of execution, the review process transforms. You're not reading 500 pages hoping to catch an error—you're reviewing the three exceptions that occurred during the batch: the times someone had to override a warning, the parameters that approached limits, the decisions that required judgment. Review time drops from weeks to hours, and review becomes meaningful. Instead of checking that data was transcribed correctly, reviewers focus on the decisions that actually matter.
Equipment integration and material verification
Manufacturing equipment generates enormous amounts of data that traditionally gets ignored or manually transcribed. A bioreactor logs temperature every second—tens of thousands of data points per batch—yet paper-based operations reduce this to "temperature maintained within range" with a single checkmark. Seal integrates directly with bioreactors, fillers, autoclaves, balances, and other equipment to capture process data automatically.
Temperature profiles, pressure readings, weight measurements, timing data—all of it flows into the batch record without anyone typing anything. The data that enters your quality record is the data that came from the equipment, with complete traceability and no transcription errors. When an auditor asks "what was the temperature at 14:32:07?", you have the answer—because the bioreactor told you, not because someone wrote it down.
Material dispensing is where many manufacturing errors originate. The wrong material, the wrong lot, the wrong amount—any of these can destroy a batch. Operators scan material barcodes and the system verifies it's the correct material and lot. Balances connect directly so weights are captured automatically. The system calculates required amounts based on the batch size and enforces tolerances. If something is wrong, the operator knows immediately—not hours or days later when someone reviews the paperwork.
When an operator scans a material barcode, the system verifies five things in milliseconds: Is this the right material? Is this the right lot? Is it released for GMP use? Is there enough quantity? Has it expired? If any check fails, the operator cannot proceed—the system tells them exactly what's wrong. When materials pass verification, the system captures the lot number in the batch record and decrements inventory automatically. No separate inventory transaction. No reconciliation at end of day. The batch record and inventory system stay synchronized because they're the same system.
Operator qualification and cleaning verification
An untrained operator executing a critical step is a deviation waiting to happen. In paper-based systems, you discover the problem weeks later during batch review: "Wait, did Martinez complete their aseptic technique requalification before this fill?" With Seal, you never get to that question—because an unqualified operator cannot execute the step in the first place.
Each step in your batch record can require specific training curricula. "Aseptic Fill Step 3" might require qualification on the specific filling line, current gowning certification, and completion of the aseptic technique SOP. The operator scans their badge. In milliseconds, the system checks their training record against all requirements. If they're qualified, they proceed. If they're not, they can't—the system won't let them start the step. When training expires, the system knows instantly. An operator who was qualified yesterday but whose annual recertification lapsed at midnight cannot execute those steps today. Compliance is continuous, not point-in-time.
Between batches, equipment must be cleaned. Between products, lines must be cleared. Seal treats cleaning as a first-class workflow, not a checkbox on a paper form. Cleaning records link to three things: the equipment cleaned, the batch that dirtied it, and the batch that will use it next. Swab test results flow in from LIMS—if the rinse water conductivity is out of spec, you know before the next batch starts, not after it's contaminated. Hold time calculations enforce your validated cleaning hold times. If your validation says equipment must be used within 72 hours of cleaning, the system knows—and won't let you start a batch on equipment cleaned 73 hours ago.
Line clearance for product changeover follows a structured checklist. The system won't allow the new product to start until every clearance item is complete. This isn't paperwork—it's a gate. The contamination events that destroy batches and careers happen when someone starts a new product before the line is truly clear.
In-process controls and hold times
Manufacturing isn't fire-and-forget. Critical steps require in-process checks: pH verification before proceeding, osmolality confirmation, bioburden samples, appearance checks. Seal manages the sampling workflow alongside the manufacturing workflow, ensuring checks happen when they should and results link to exactly the right step.
At Step 12, the batch record calls for a pH check. The operator takes the sample and scans the sample container. The system creates a sample record in LIMS—with the batch number, step number, timestamp, and operator ID already populated—and pauses the batch. The batch cannot proceed to Step 13 until LIMS returns the result. If pH is within range, the result populates in the batch record and the batch proceeds automatically. If it's out of range, the system flags the exception and routes to your defined escalation path.
Hold times are enforced automatically. If your process validation established that Step 15 must start within 4 hours of Step 12 completing, the system tracks this from the moment Step 12 completes. At 3 hours, warnings appear. At 3.5 hours, the warning escalates to supervisors. At 4 hours, if Step 15 hasn't started, the batch cannot proceed without documented justification and approval. This isn't nagging; it's enforcement of the constraints your validation established.
Visibility, genealogy, and traceability
Stop guessing where your batches are. Seal turns your facility into a glass box, providing real-time visibility into every aspect of manufacturing operations.
You know exactly which step every batch is at. You know which rooms and equipment are occupied and when they'll be available. You can see a batch falling behind schedule and intervene before it becomes a problem. Every batch has a story, and with Seal, you can tell it.
Click any finished product and see its complete genealogy: every raw material lot that went into it, every piece of equipment it touched, every operator who worked on it, every environmental condition it experienced, every test result from QC. Forward traceability works too: click any raw material lot and see every batch that used it, every product that contains it. When a supplier issues a recall notice at 3pm, you know by 3:05pm which batches are affected. When a customer reports a quality issue, you can trace back to exact conditions—not "around January" but "Lot FP-2024-041, manufactured 2024-01-15, using API lot RM-892 from Acme, mixed on equipment MX-001 for 2 hours at 200 RPM." This isn't archaeology—it's a query.
One platform, GxP compliance by architecture
Because your MES lives on the same platform as your LIMS and QMS, the handoffs that create delays in traditional systems don't exist. QC results from the LIMS appear directly in the batch record as testing completes—no waiting for someone to email a spreadsheet. Deviations open in the QMS directly from the manufacturing step that triggered them, with full context captured automatically. For batch release, all the information needed to make a release decision is in one place.
21 CFR Part 11 and EU Annex 11 compliance isn't a feature to add—it's an architecture to build on. Every signature requires two-component authentication with re-authentication at the moment of signing. The signature captures who signed, when they signed, and what they were signing—the exact record state at signature time. The meaning of the signature is explicit: "I performed this step" differs from "I verified this step" differs from "I approved this batch." Signatures are linked to training records and role assignments—an operator can sign that they performed a step but cannot sign that they verified it unless they have the verifier role.
The audit trail is immutable by design—ALCOA+ compliant from architecture, not policy. Every action in the system is logged with user, server timestamp, and before/after values. You cannot delete audit trail entries. You cannot modify them. There is no "admin override" that bypasses the audit trail. Data is encrypted in transit and at rest, with SOC 2 Type II certification and records retained for a minimum of three years beyond your subscription.
Implementation that respects reality
Timing matters. If you're in early-stage R&D or Phase 1 with infrequent batches and constantly changing processes, an MES might constrain you—stick to a flexible ELN and paper until your process stabilizes. If you're entering Phase 3 or commercial launch, implementing an MES during a pivotal trial is high-risk. The sweet spot is Phase 2 or early Phase 3: your process is stabilizing, your volume is increasing, and you need to lock down compliance before commercialization.
You've seen the legacy MES implementations—eighteen-month timelines that stretch to three years, budgets that double, go-lives pushed quarter after quarter. Seal works differently. You start by digitizing a single unit operation—weigh and dispense, perhaps, or a critical filling step. Within days, not months, your operators execute that step digitally. Your QA team sees what review by exception actually looks like. You validate one workflow, not an entire system. You see the value before you've bet the company on it.
Converting paper batch records to digital workflows shouldn't mean re-typing every step. Drop your existing master batch records—Word documents, PDFs, even scanned paper—and AI extracts the structure. A 50-page paper MBR becomes a structured digital workflow in hours, not weeks. AI identifies unit operations, process parameters, material requirements, equipment specifications, and verification steps. Drop your process validation reports and AI extracts the proven acceptable ranges. The pattern is consistent: AI extracts, you review the changeset, approved data enters the system. Your process experts validate the extraction—they're not typing it.
Every batch your team runs today generates data that could drive faster release, fewer deviations, and better process understanding. Paper buries that data in binders. Legacy EBR systems trap it in rigid forms. Seal liberates it. Your process, digitized. Your compliance, enforced. Your data, accessible. Your team, focused on what matters.
Capabilities