Process Development
Develop. Promote. Execute.
Development work in an execution system. When you go to GMP, you promote—not re-author. Tech transfer becomes configuration, not translation.
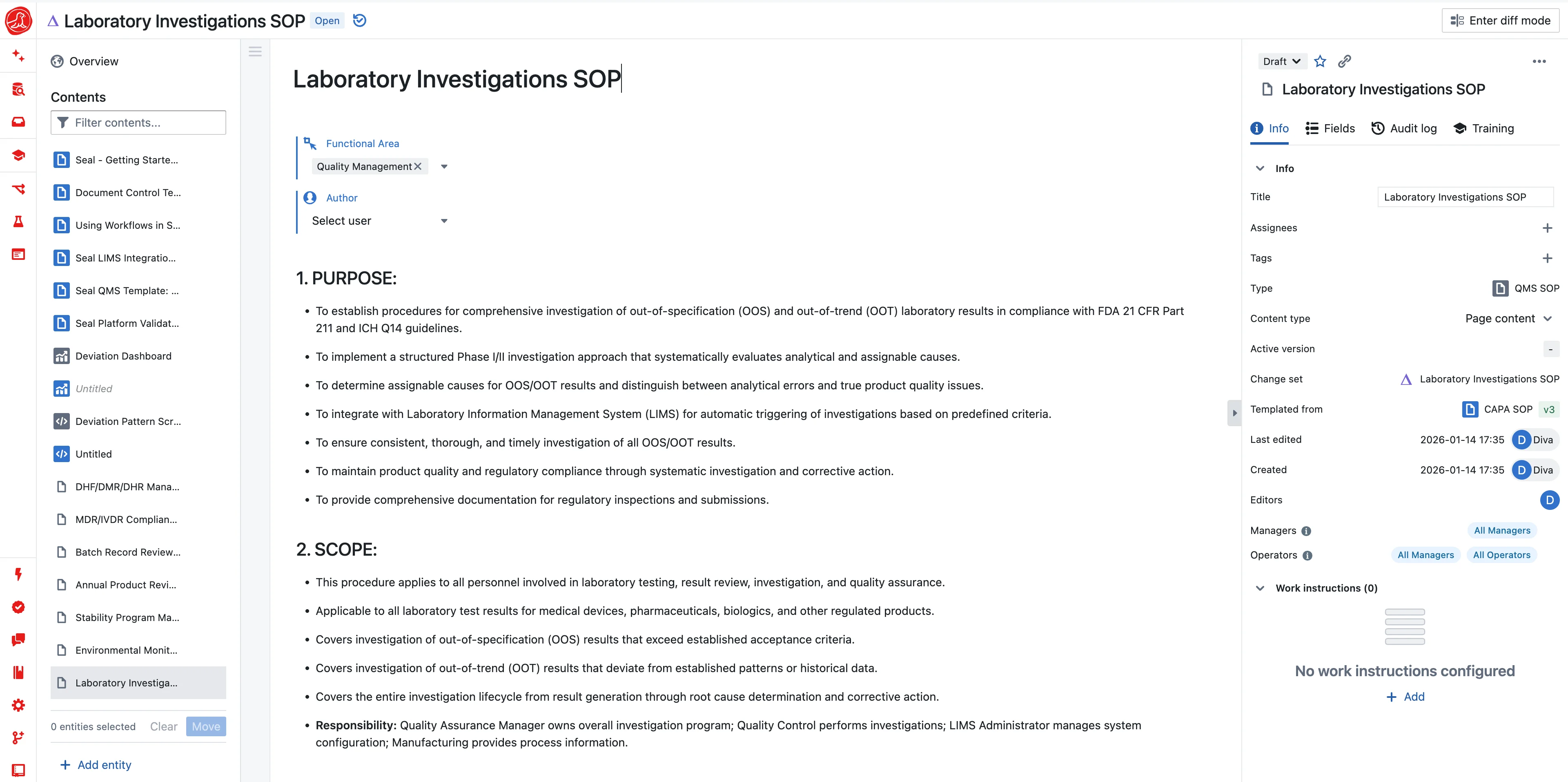
The tech transfer that took eighteen months
The process worked at bench scale. It worked at pilot. The development team documented everything—thousands of pages of reports, protocols, data. Then came tech transfer.
Manufacturing couldn't run from development's documents. The batch record had to be written from scratch. Parameters that were obvious to the development team were buried in slide decks. When questions arose, the scientists who knew the answers had moved on. The "knowledge transfer" was really knowledge excavation. Eighteen months from "process locked" to "first GMP batch."
This isn't a failure of documentation. It's a failure of architecture. Development and manufacturing are running on different systems with different data models. The translation layer is humans reading documents and typing into new systems. That's where knowledge dies.
Development in an execution system
Most development teams work in documentation systems. They write protocols, execute experiments, generate reports. The work is real. The data is real. But the output is documents—PDFs, Word files, PowerPoints—that have to be translated into manufacturing systems later.
Seal is an execution system, not a documentation system. When a development scientist defines a unit operation, they're creating a reusable, version-controlled asset—not writing a document. When they capture a process parameter, it's structured data with context—not a number in a spreadsheet. When they run an experiment, the execution is recorded with the same rigor that GMP will require.
This isn't about forcing GMP compliance onto development. Development needs flexibility. Scientists need to iterate, explore, adjust. Seal provides that flexibility—but the flexibility happens within a structured framework, not outside of it.
Flexible when you need it, rigorous when you don't
Development workflows are different from manufacturing workflows. Scientists need to deviate, try alternatives, document observations that weren't planned. Forcing them into rigid GMP-style execution kills productivity and misses insights.
Seal handles this by separating structure from enforcement. The structure is always there—unit operations, parameters, materials, equipment. But enforcement is configurable. During early development, parameters are captured but not enforced. Deviations don't require approvals. The scientist has freedom to explore.
As the process matures, controls tighten. Parameter ranges narrow. Deviations require documentation. By late-stage development, the process runs with near-GMP rigor—not because someone mandated it, but because the process is ready for it.
Promote, don't re-author
The tech transfer problem isn't a "documentation problem" to solve with better templates. It's an architecture problem. If development and manufacturing are different systems, translation is unavoidable.
Seal eliminates translation by keeping development and manufacturing on the same platform. The process you develop is the process you promote. The unit operations, parameters, equipment requirements, material specifications—all of it carries forward. You're not re-authoring a batch record from development documents. You're configuring the validated version of the process you already built.
What changes during promotion? Enforcement tightens. Approvals are required. Deviations route to quality systems. But the process definition—the actual work—doesn't change. That's what "digital tech transfer" means. Not sending files. Promoting assets.
FDA is looking further back
Regulatory expectations have shifted. FDA doesn't just want to see your GMP manufacturing records. They want to understand your process—why you chose those parameters, how you know they're critical, what happens when they drift. That understanding lives in development.
If development data lives in a documentation system, answering these questions means archaeology. Finding the right reports. Hoping the scientist documented their reasoning. Reconstructing context from fragments.
If development data lives in an execution system—the same one you're running GMP on—the questions answer themselves. Click a CPP and see its history: the experiments that established it, the characterization that confirmed it, the range that emerged from the data. The regulatory story isn't assembled. It's inherent in the data structure.
Upstream, downstream, analytical
Process development isn't monolithic. Upstream teams optimize cell culture and fermentation. Downstream teams develop purification. Analytical teams create the methods that measure it all. These aren't separate processes—they're connected parts of one process. But most tools treat them as silos.
Seal models the process as it actually exists. Upstream unit operations connect to downstream unit operations. The output of fermentation is the input to purification. Analytical methods tie to both. When upstream changes affect downstream, the system shows the impact—because it's one connected model, not three separate documentation projects.
Scale-up without surprises
The process that works at 2L doesn't automatically work at 2000L. Scale-up is where development processes break—and where tribal knowledge matters most. "We always do X at large scale" isn't written anywhere. Until it's missed, and the batch fails.
Seal captures scale models as structured relationships. Parameters at bench scale link to predictions at pilot and commercial. When a scientist establishes a mixing time at bench, the system can project what that means at manufacturing scale—not through magic, but through the scale correlations your team has built.
This doesn't replace engineering judgment. It augments it with data. The engineer can see not just "what's the mixing time?" but "what was the mixing time at every scale this process has run, and what happened to product quality at each?"
Process characterization built in
Understanding your process isn't a separate activity from developing it. Every experiment generates data about parameter impacts. Every run reveals relationships between inputs and outputs. Process characterization is the systematic capture and analysis of these relationships.
Seal treats characterization as a first-class capability. Parameters link to quality attributes. Experiments are designed to explore the design space. The data that establishes your critical process parameters and proven acceptable ranges is the same data you captured during development—structured, linked, and queryable.
When you need to define your design space for regulatory submissions, you're not reconstructing it from reports. You're querying it from the data. The characterization is already done. It just needs to be presented.
The lifecycle advantage
Most organizations treat development, tech transfer, manufacturing, and commercial support as phases with handoffs. Each handoff loses information. Each system transition requires translation. By the time a product is commercial, understanding the original development decisions requires archaeology.
Seal eliminates the phases by eliminating the system boundaries. Development happens on Seal. Tech transfer is promotion within Seal. Manufacturing executes on Seal. Commercial lifecycle management—process changes, deviations, continuous improvement—happens on Seal. The data is continuous. The context is preserved. The knowledge compounds instead of eroding.
This isn't just convenience. It's a competitive advantage. Companies that maintain process knowledge can respond faster to problems, improve processes more effectively, and defend their products to regulators with confidence. The platform that holds development also holds manufacturing. The platform that knows why also knows how.
Capabilities