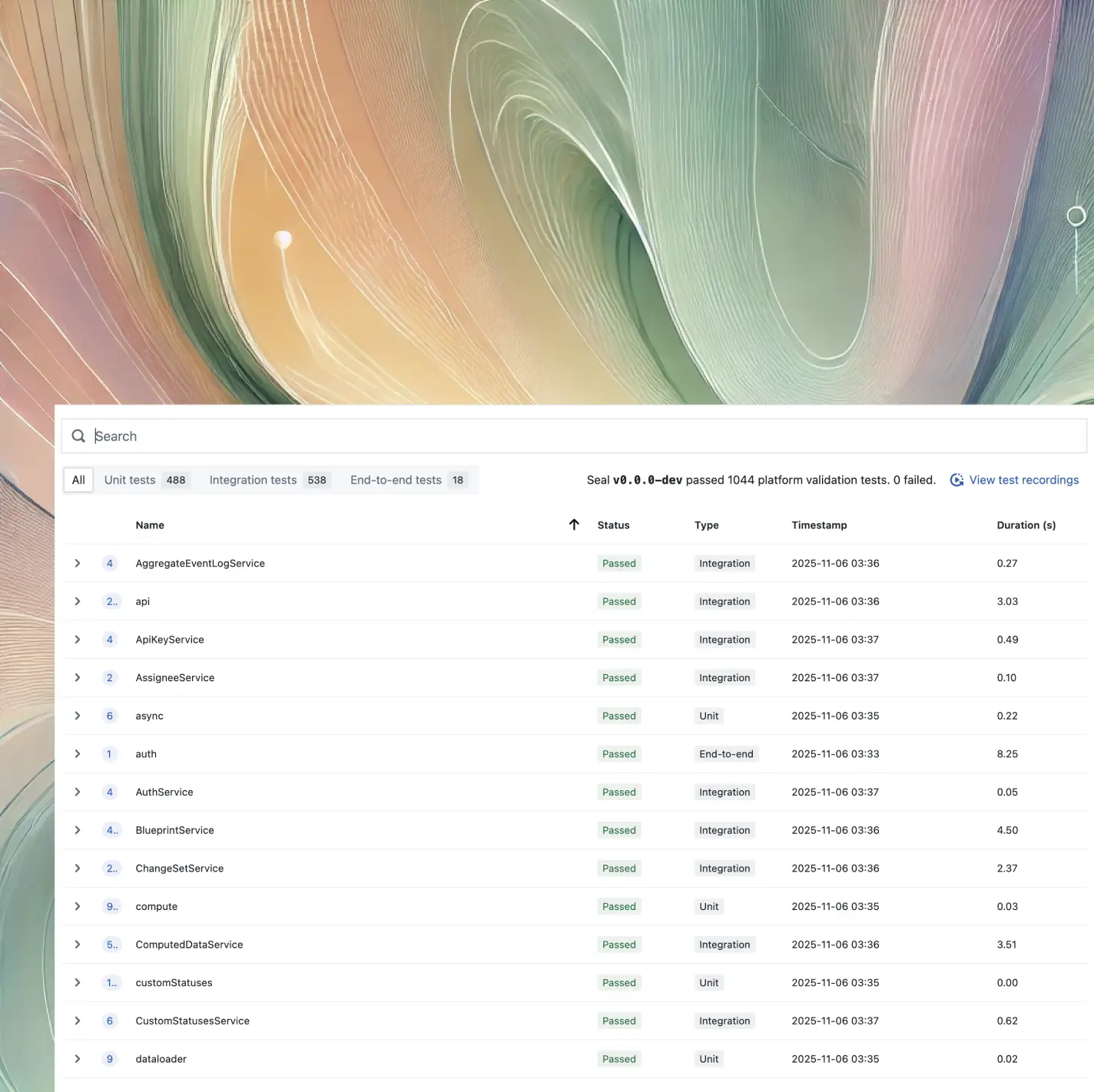
Connect validation across systems
Built to GAMP 5 2nd Edition and the FDA's 2022 CSA Guidelines, ensuring your validation processes meet regulatory standards while maintaining efficiency and traceability
vlms
Validation lifecycle management systemqms
Quality management system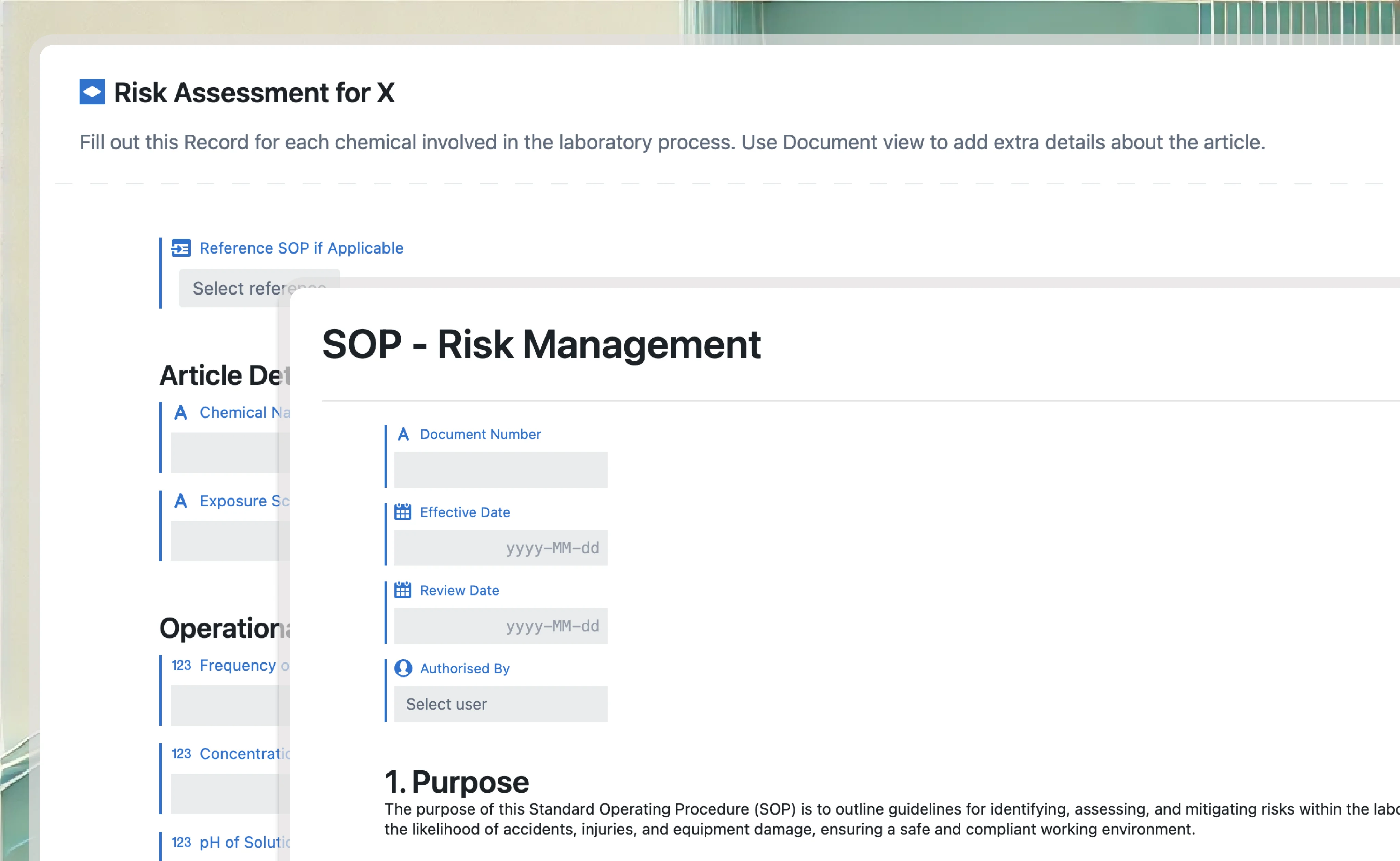
Integrate VLMS with QMS to streamline validation processes within quality management workflows. Connect validation records to quality events, ensuring comprehensive compliance and traceability across both systems
eln
Electronic laboratory notebook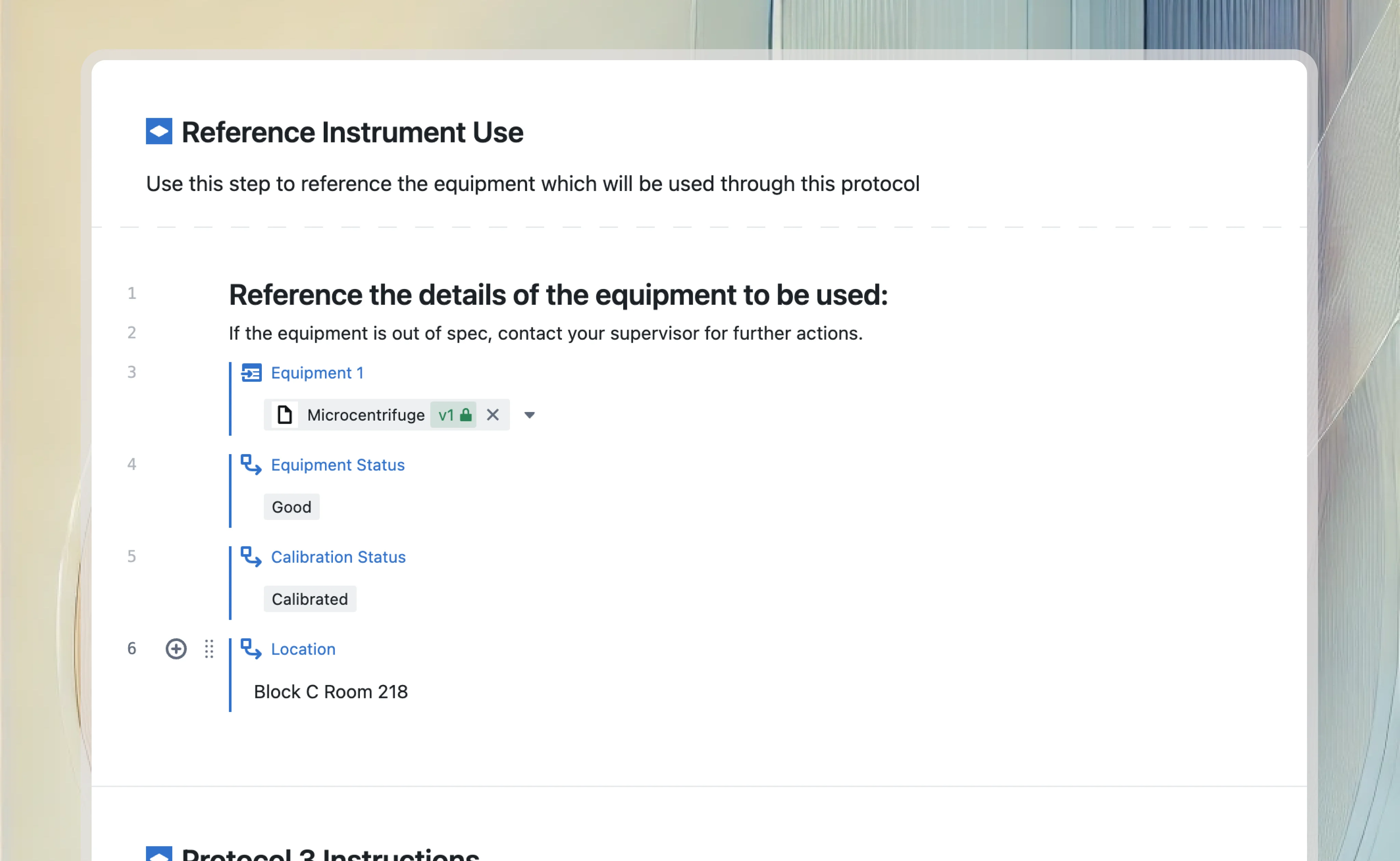
Connect VLMS with ELN to validate experimental workflows and ensure compliance. Link validation records to experimental data, maintaining traceability from development through validation
lims
Lab information management system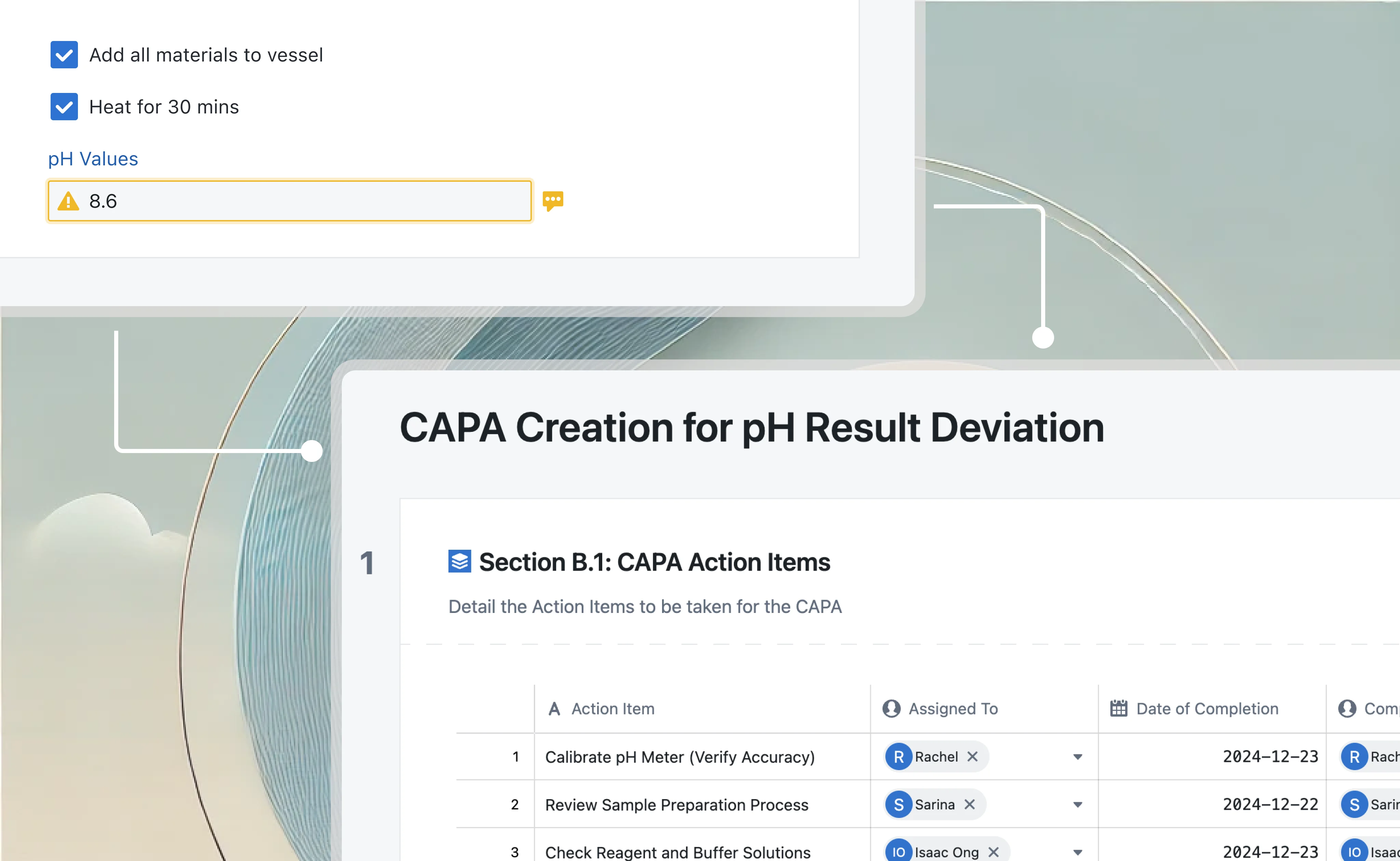
Integrate VLMS with LIMS to validate laboratory testing processes. Connect validation records to sample and test data, ensuring compliant and traceable laboratory operations
mes
Manufacturing execution system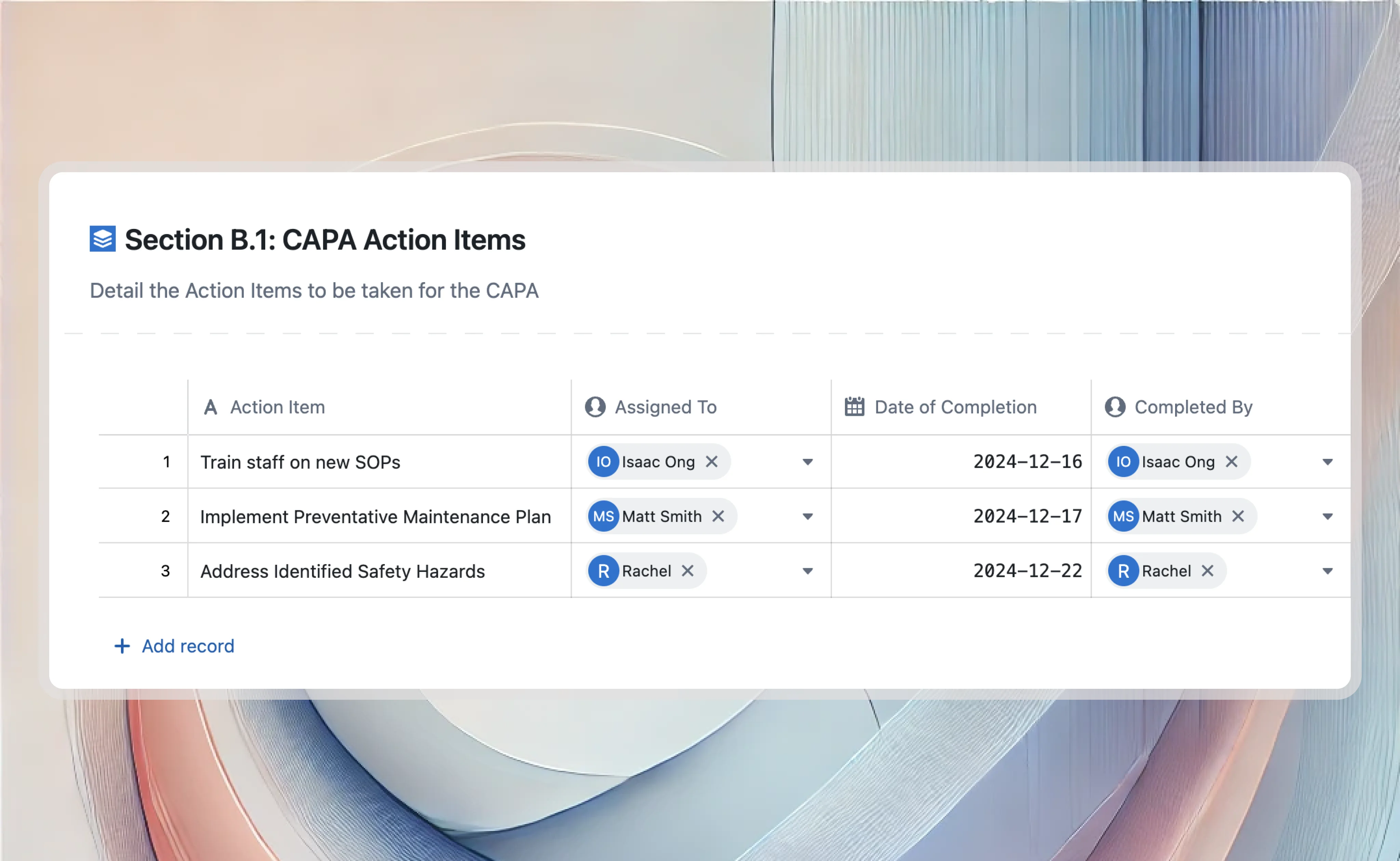
Link VLMS with MES to validate manufacturing processes and batch records. Connect validation activities to production workflows, ensuring compliance throughout the manufacturing lifecycle

Integrate VLMS with QMS to streamline validation processes within quality management workflows. Connect validation records to quality events, ensuring comprehensive compliance and traceability across both systemsLearn more >