Seal Platform
You change lives. Seal changes how fast.


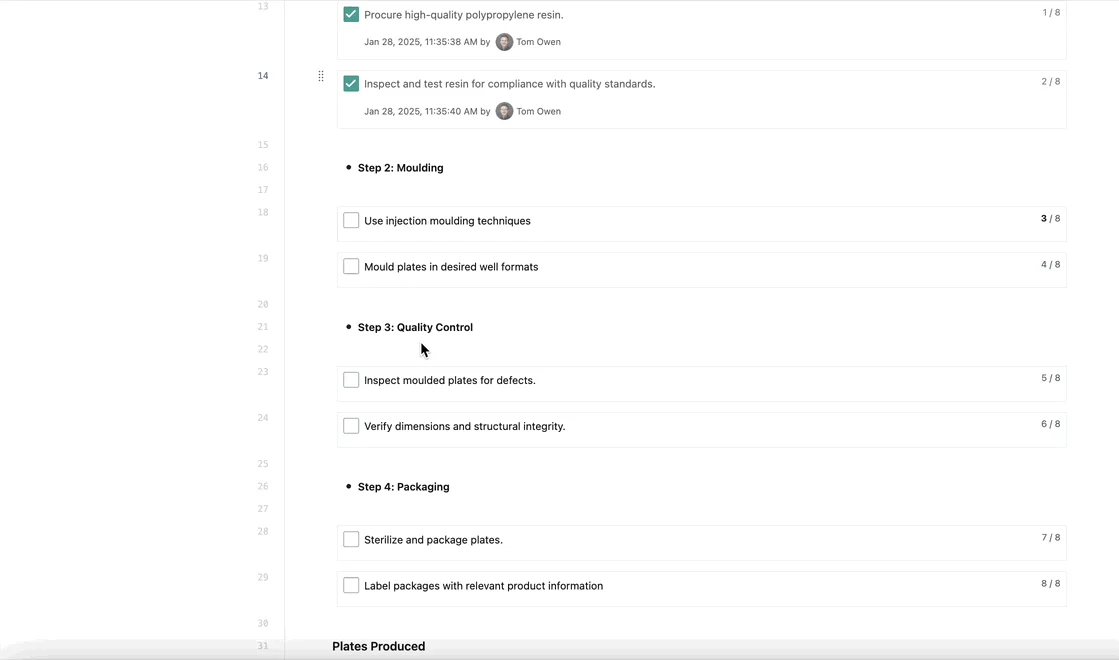
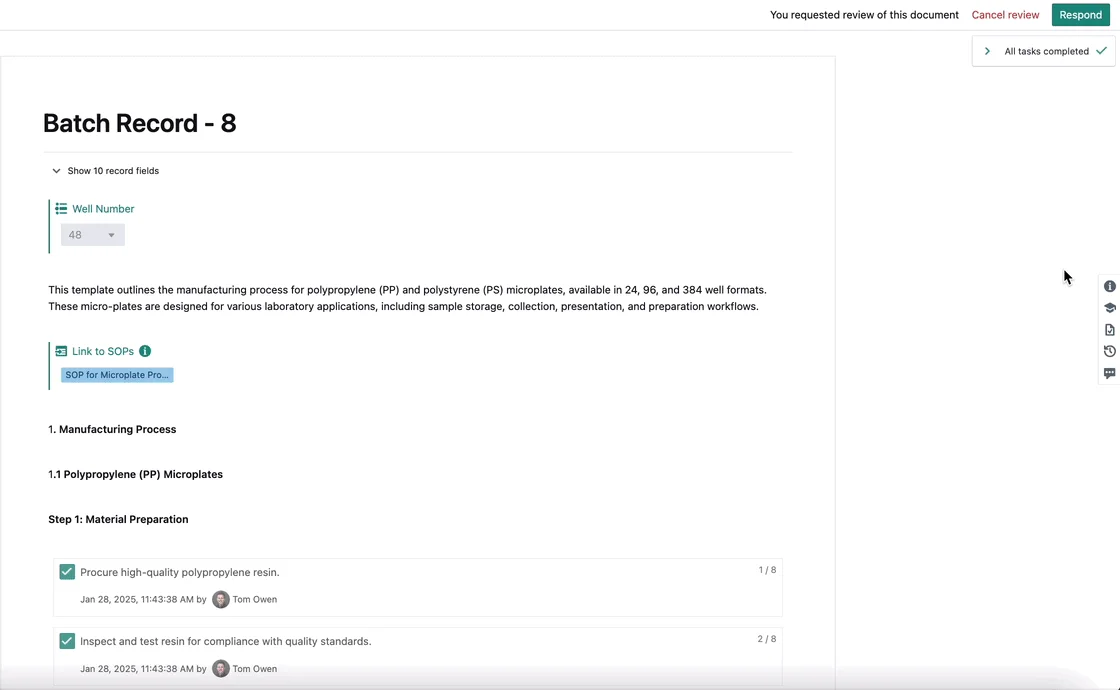
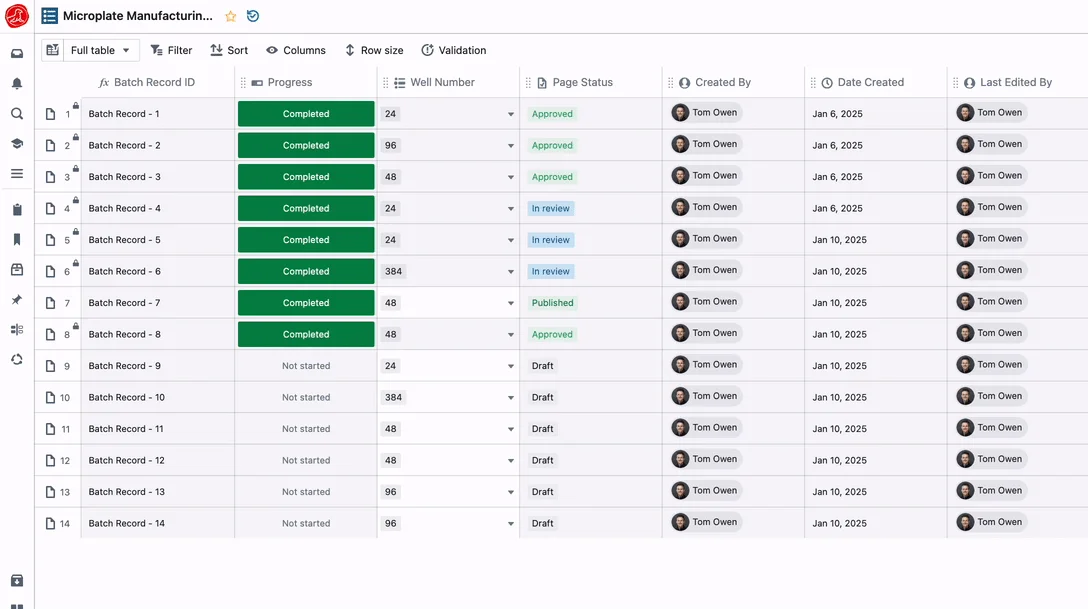
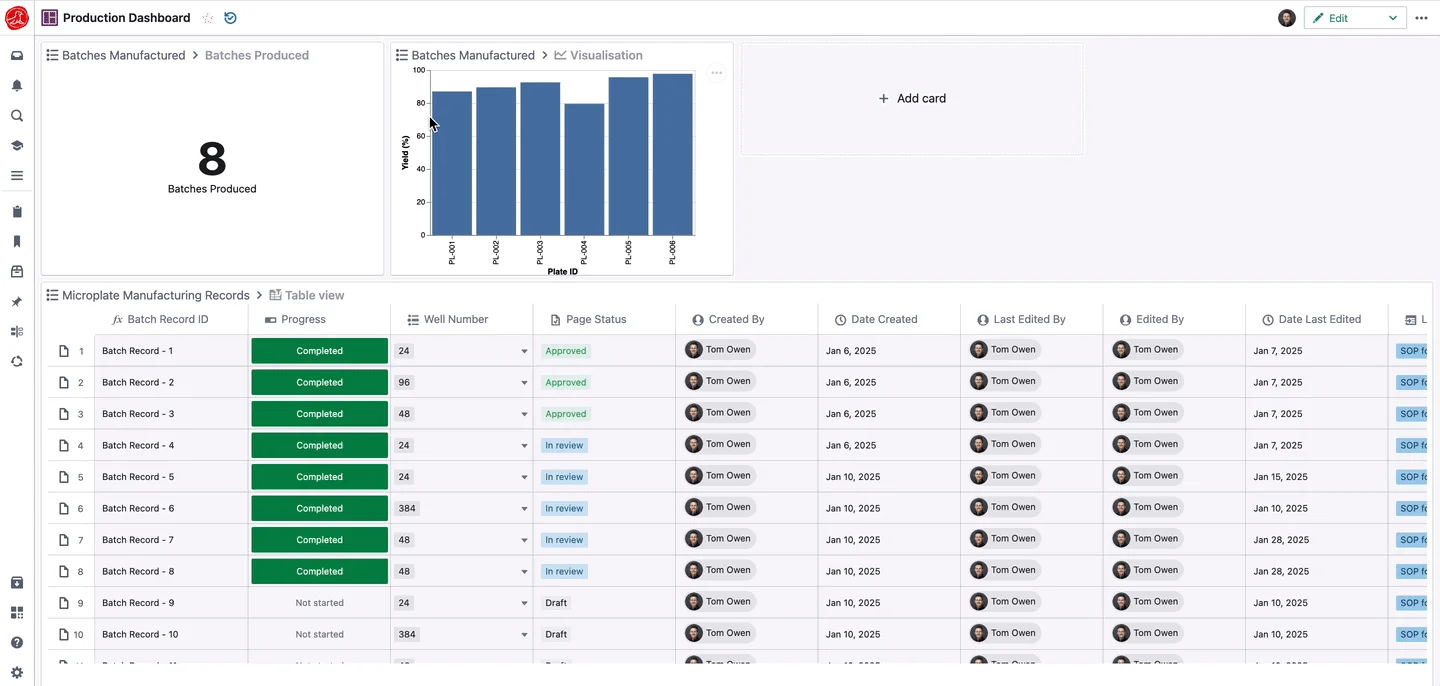
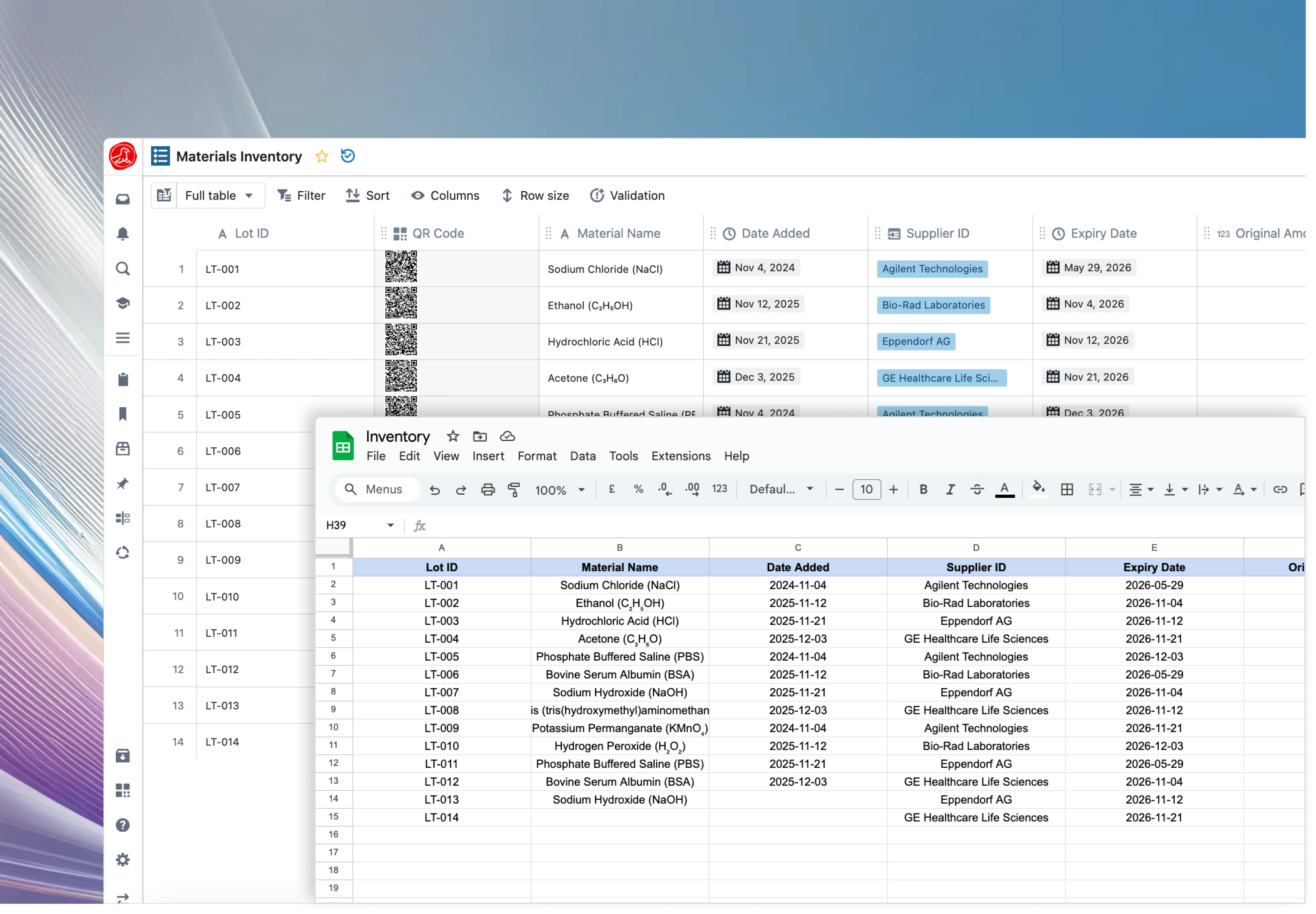
Continuously ingest data from common spreadsheet formats. Seamlessly map real-time values (e.g., production yields) into interactive dashboards for immediate analysis.
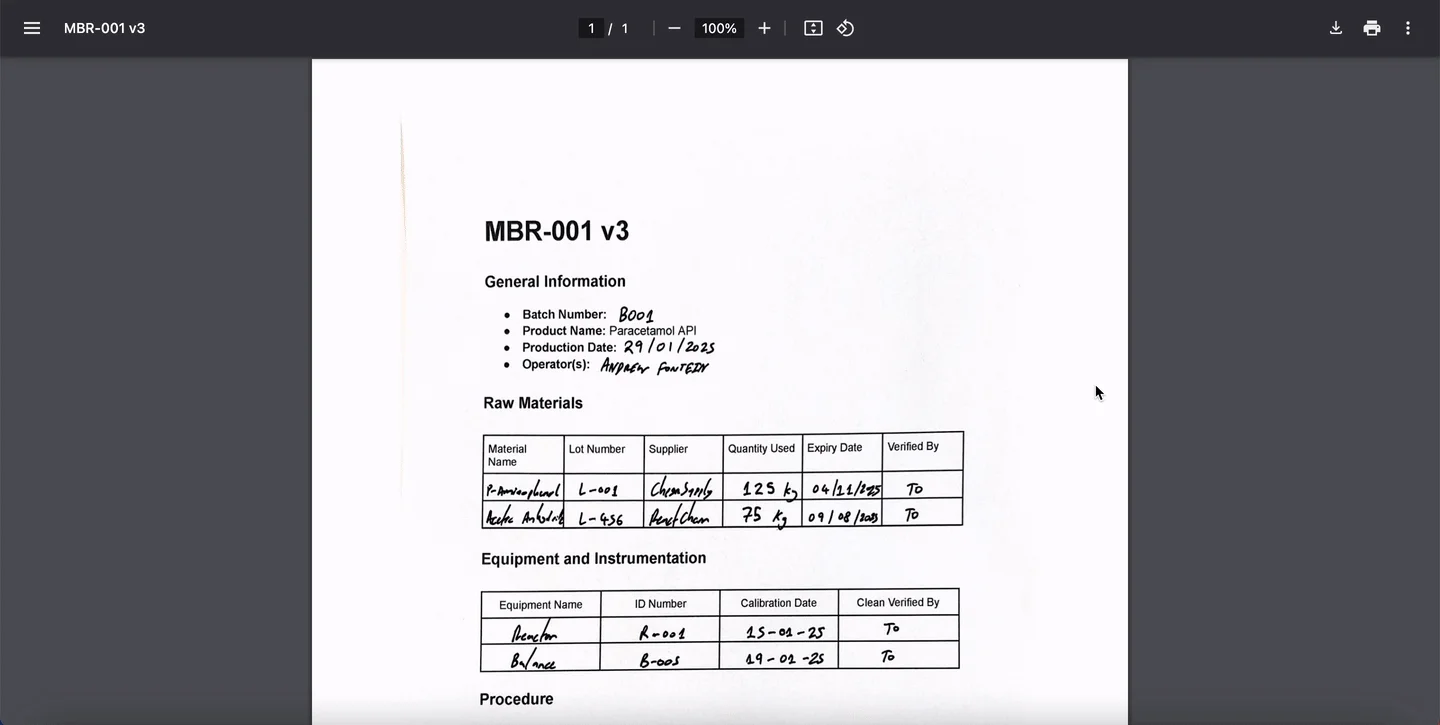
Pull data directly from lab instruments—such as pH meters, spectrophotometers, or PCR machines—reducing manual input and capturing a complete picture of quality within a single system.
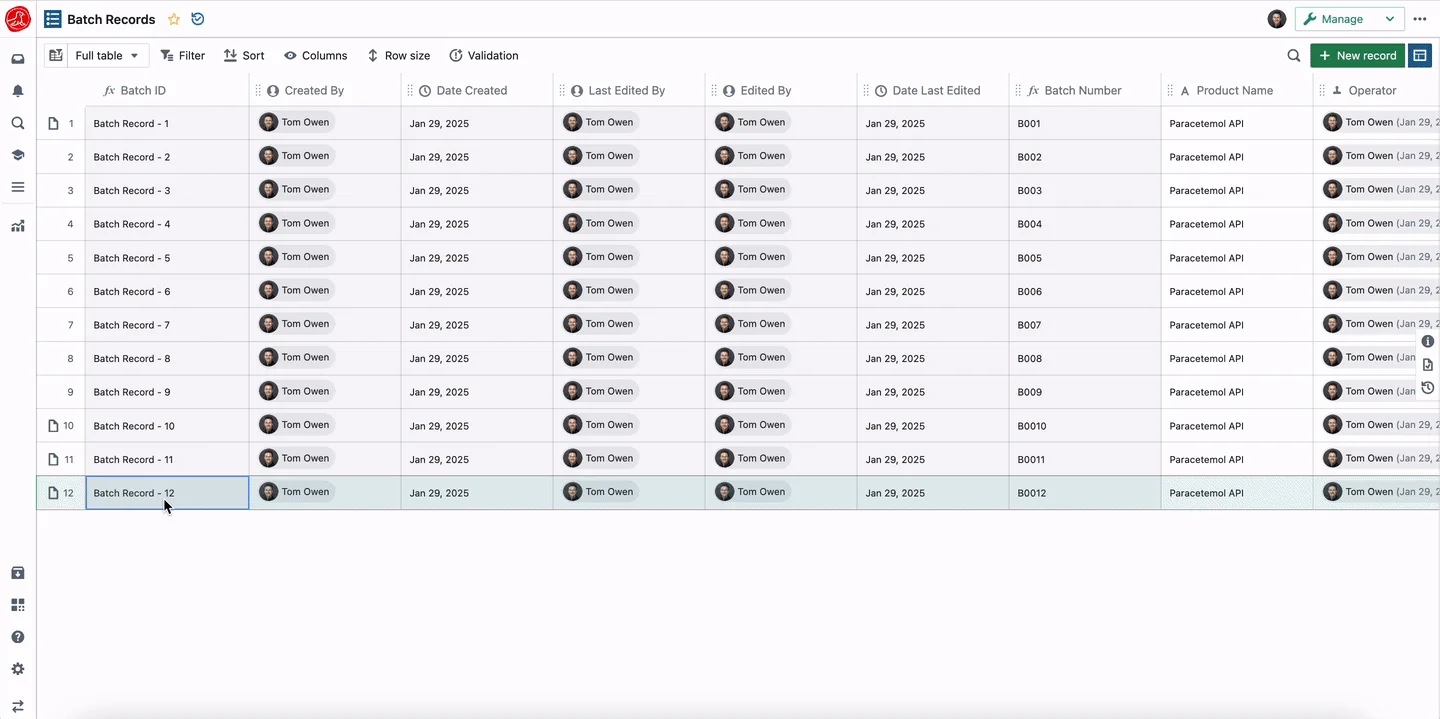
Sync essential manufacturing and resource planning parameters automatically. Minimize transcription errors and ensure that quality data ties back to each step along your process lifecycle.
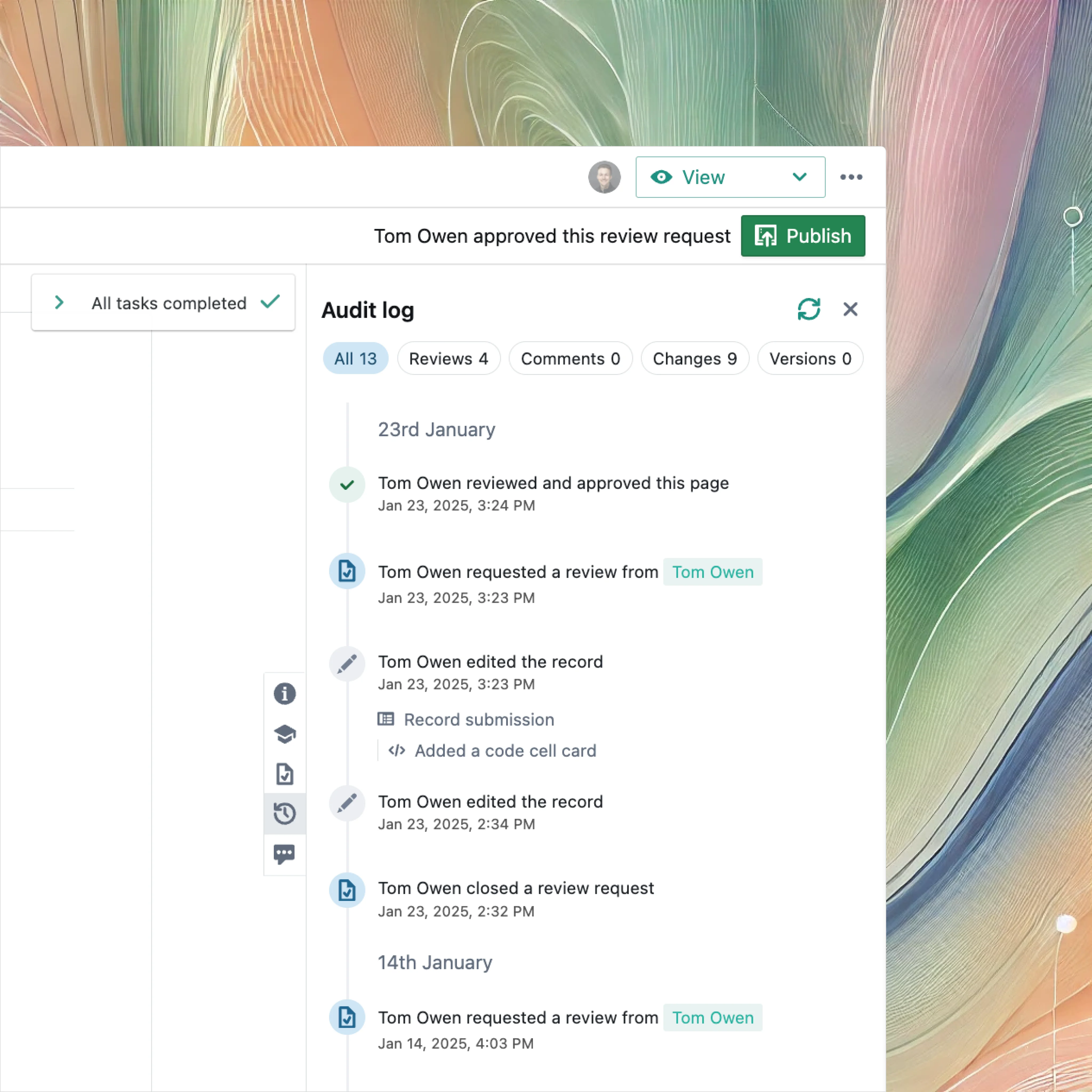
Integrate with GitHub and other systems to maintain complete traceability for regulated software development such as SAMD.
Tamper-proof audit trails, legal and secure e-signatures, robust authentication mechanisms and encrypted data.
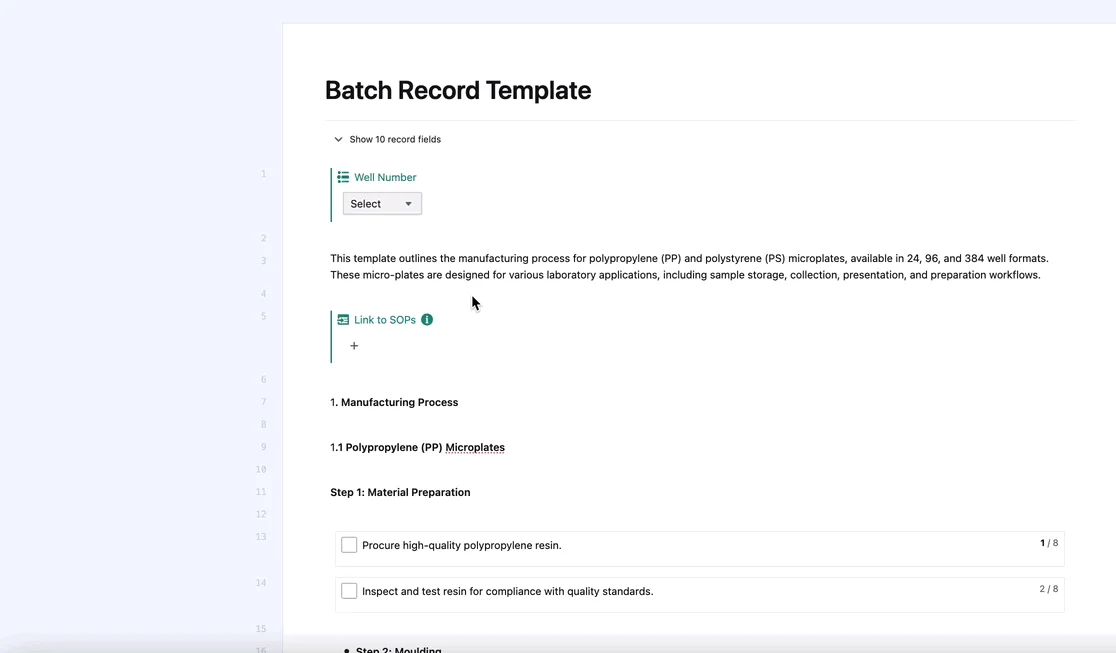
Get started with industry-prescribed template packs and best practices - fast forward your journey towards your particular ISO compliance standard.
Comprehensive GxP validation and change-control system to simplify IQ, OQ, and PQ and enable continuous improvement.