The GxP Execution System.
Unify ELN, Inventory, LIMS, MES, QMS, and Clinical on one AI-native platform. Friction is not rigor. Go live in 48 hours.
"We've reduced operator paperwork time by over 80%."
Blueprints
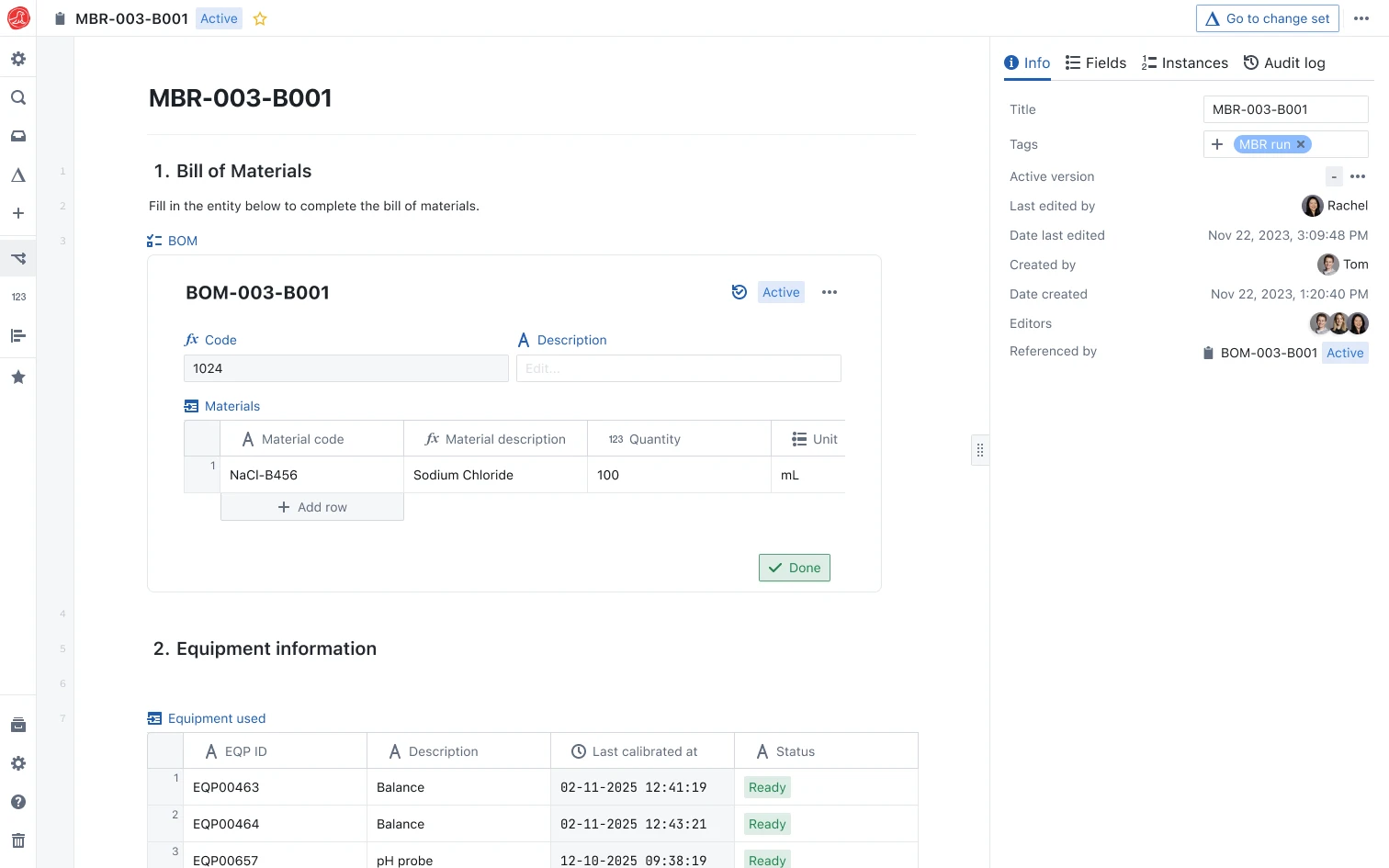
Digital batch records that enforce compliance and accelerate release.

Unify document control, training, and quality events into a single, audit-ready platform that your team will actually love.
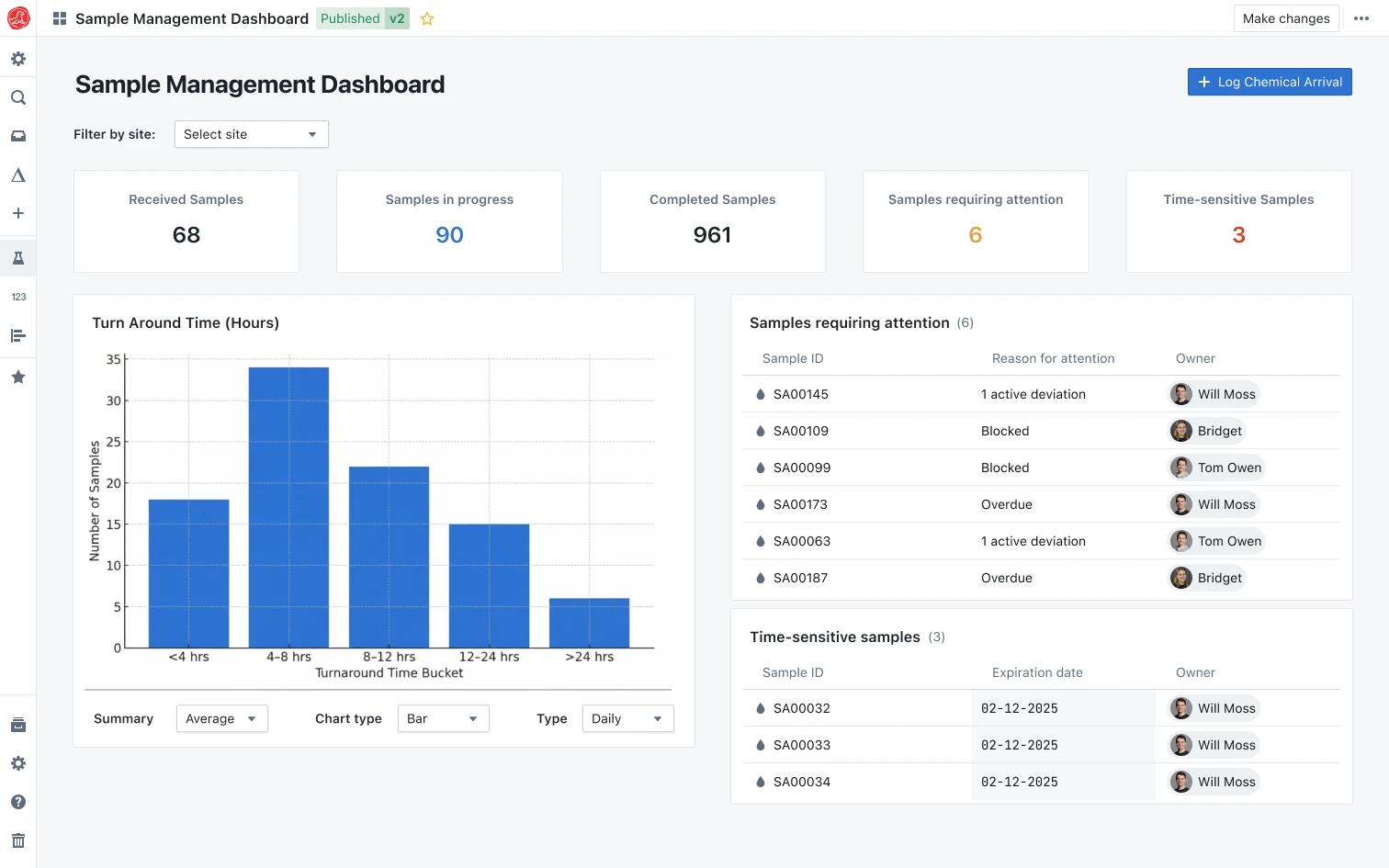
The batch waited three weeks. Manufacturing took one day. QC paperwork took twenty. Release testing that clears batches in days, not weeks.
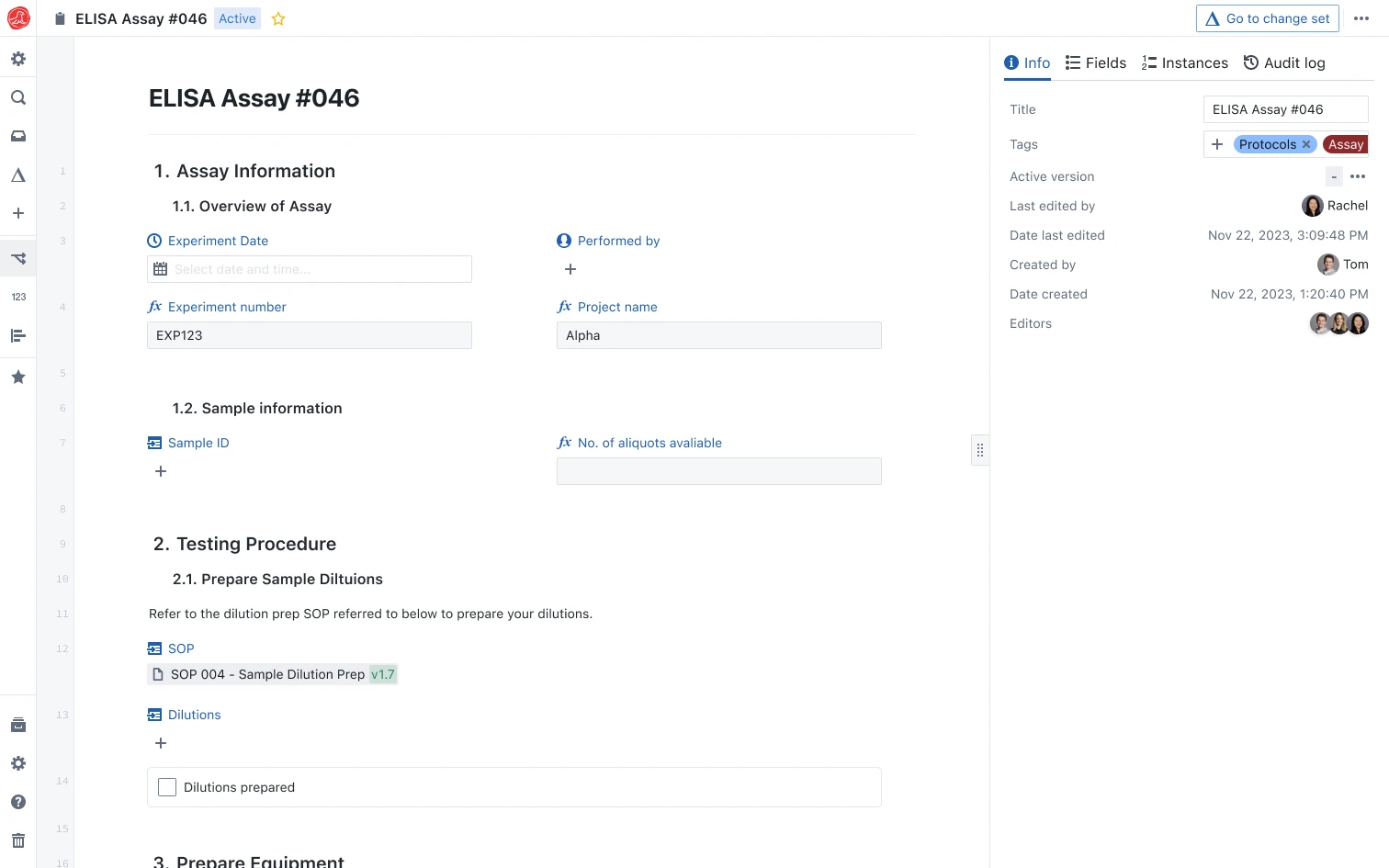
Turn experiments into structured data AI can actually use.
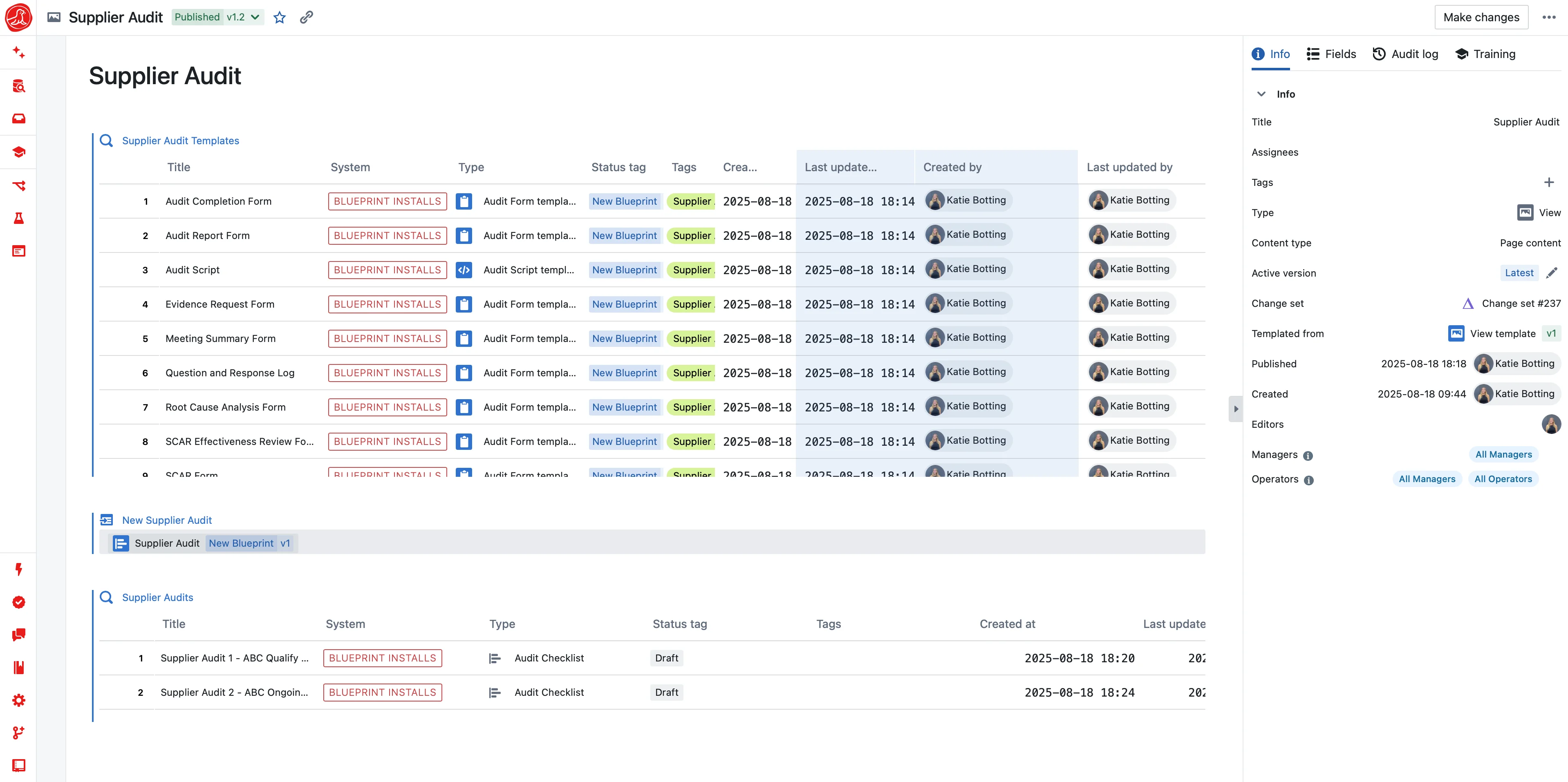
Container-level tracking with status enforcement. No more stockouts. No more expired-material deviations. No more 'I thought we had some.'
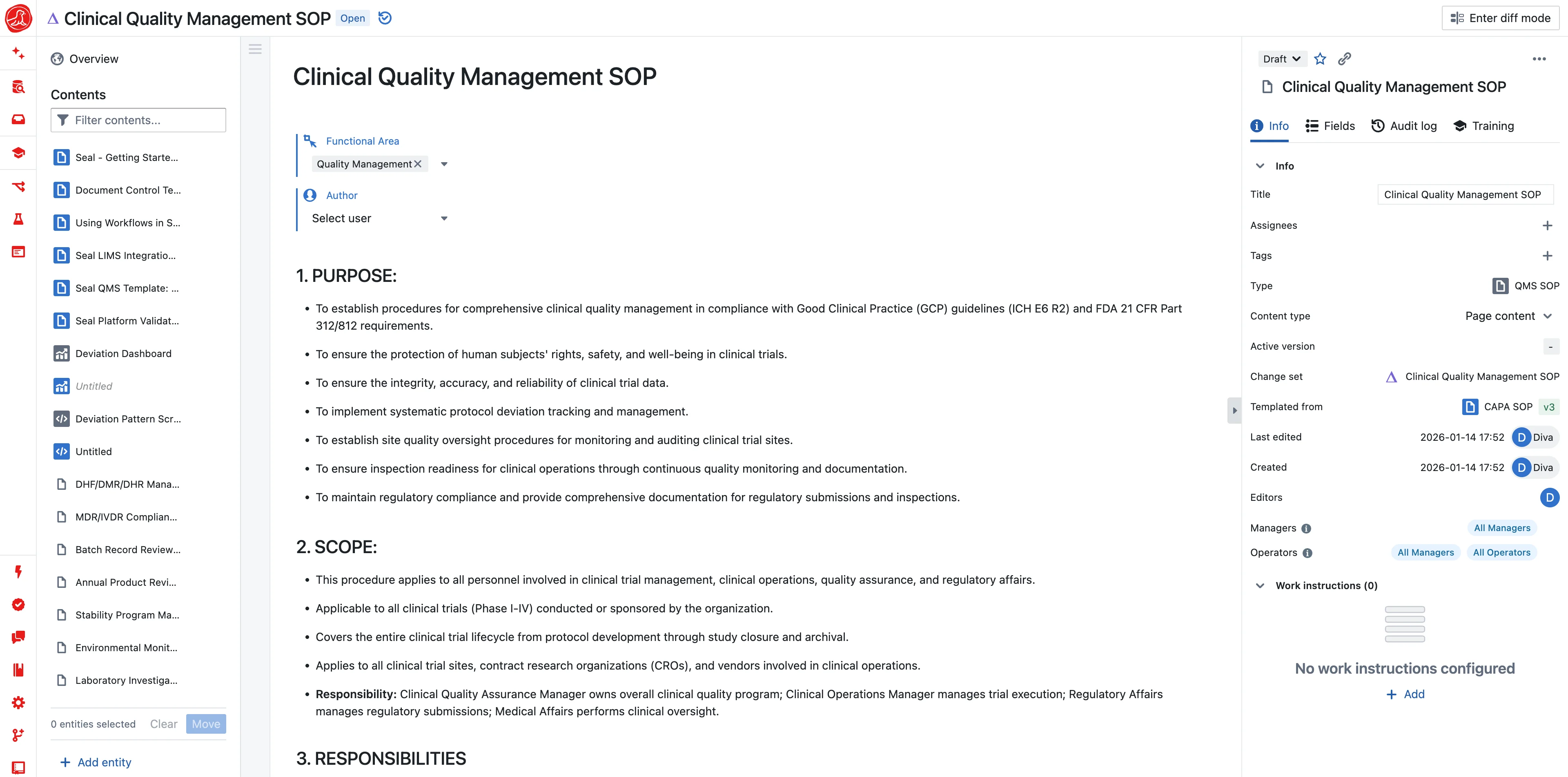
EDC, CTMS, and eTMF share one data model. Sites enter data once. Documents file themselves. Queries resolve in context.