For decades, Quality Management Systems (QMS) have been the cornerstone of quality and compliance in the life sciences industry. However, as technology advances and the pace of innovation accelerates, these traditional systems are increasingly becoming relics of the past. It’s time for the industry to embrace a more integrated and agile approach. Here’s why the old QMS is dead and why GxP OS is the future.
The birth and evolution of eQMS
Let’s take a trip back to the 1950s. Imagine a laboratory filled with stacks of paper, employees tirelessly sifting through documents, and approvals moving at a snail’s pace. Shockingly, many labs still look like this today! This was the reality of paper-based QMS—labour-intensive and prone to errors.
Then came electronic Quality Management Systems (eQMS), transforming these cumbersome paper processes into fast, easy-to-track digital workflows. Documents could be stored and retrieved with a click, compliance processes became more streamlined, and traceability improved significantly.
eQMS revolutionised the way laboratories operated. The shift from paper to digital meant that what once took hours or days could now be accomplished in minutes. For example, instead of manually tracking every change to a document, an eQMS could automatically log edits, provide version control, and ensure that everyone was working with the most current information. This leap in efficiency was nothing short of transformative for the industry.
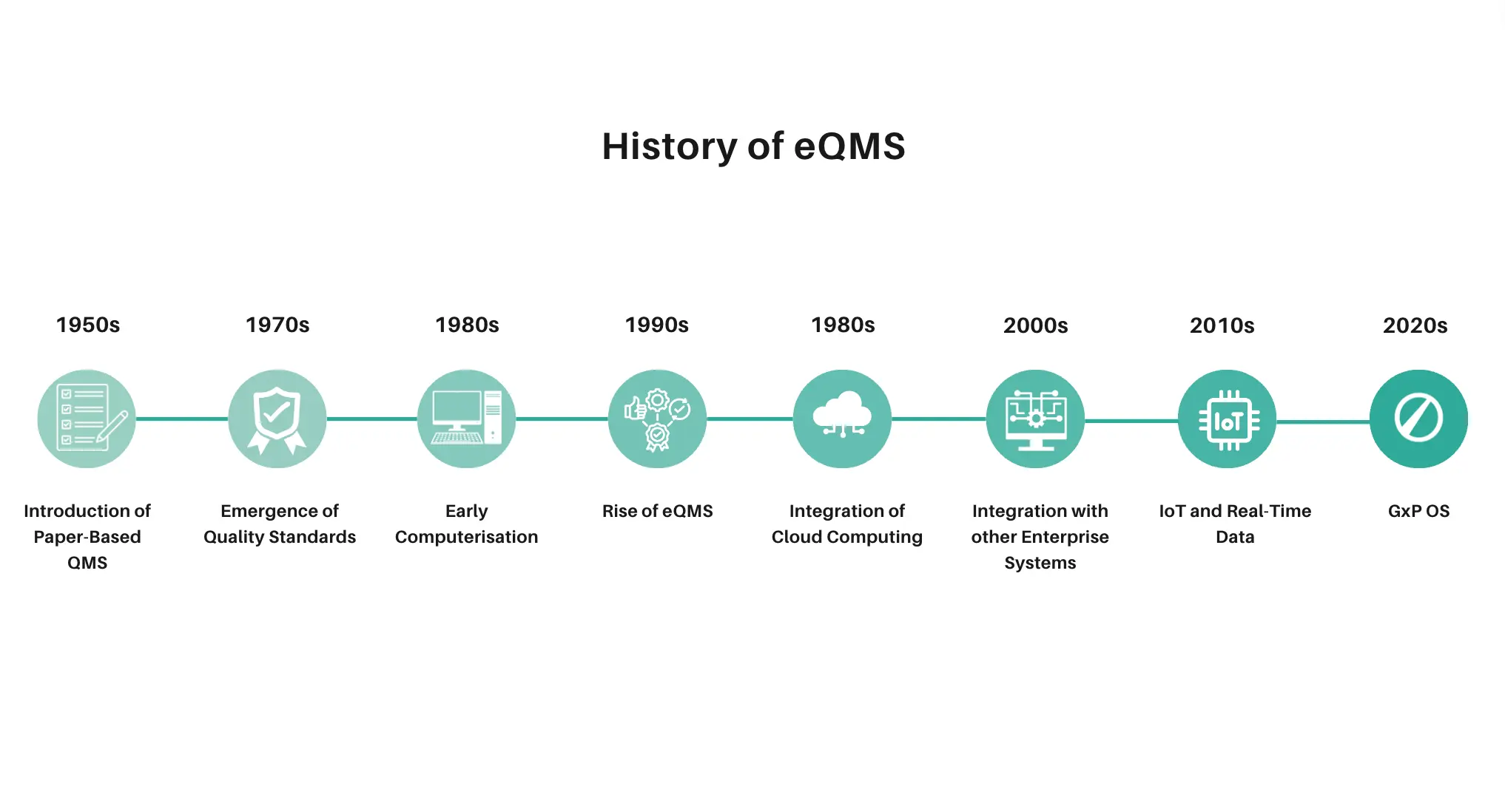
As digital technology continued to evolve, the next major leap came with cloud-based templates. Just as cloud computing transformed other sectors, it brought significant advancements to eQMS. With cloud eQMS, the core concepts of quality management remained the same, but the quality of the software, its accessibility, and its integrations improved dramatically.
Imagine a team of scientists spread across the globe, all needing to collaborate on a project. With traditional systems, this would involve countless emails, file transfers, and versioning issues. Cloud eQMS changed all that by allowing real-time collaboration, regardless of location. Teams could access the system anytime, anywhere, ensuring that they always had the latest information at their fingertips. Automatic updates kept the system current without placing a burden on IT departments.
However, despite these advancements, traditional cloud eQMS templates had their limitations. They managed documentation effectively but often did little more than just that—they documented procedures without integrating them into the systems where the actual work was executed and recorded. This separation meant that while documentation processes were streamlined, the execution of procedures often remained fragmented and inefficient.
The problem with siloed systems
For life sciences companies, it’s common for companies to juggle multiple systems to execute and document their work: Laboratory Information Management Systems (LIMS), Electronic Laboratory Notebooks (ELN), Electronic Batch Records (EBR), Manufacturing Execution Systems (MES), and even spreadsheets. Each system might function well on its own, but together they create a fragmented landscape that makes data integration and workflow management nearly impossible.
The current approach to quality management is broken.
Using different systems for different tasks slows things down and leads to mistakes. We need one system that does everything.
A Quality Management System (QMS) shouldn't be separate from the actual work being done. Instead, procedures and their execution should happen in the same place. This isn’t just about meeting compliance requirements; it’s about making sure the work is done right. Being able to reproduce results is still a major issue. In one study, up to 70% of researchers tried and failed to reproduce another scientist’s experiment results. Having all the information in one place helps keep things under control.
In biotech, for example, where processes are really complicated, it’s hard to do the same thing twice. A unified system makes sure that quality management is part of every step, making it easier to track and control every process.
The benefits of a unified system
When everything is managed and executed in one system, it creates a continuous feedback loop. Data from each task can be analysed to improve future work. For example, if a certain procedure is consistently resulting in delays or errors, the system can highlight this so that teams can make adjustments and improve the process. This way, you’re not just meeting compliance requirements; you’re actively learning and refining procedures. Quality management should improve processes over time, not just ensure compliance.
AI can make a big difference
When all data and tasks are in one system, AI can make a big difference. AI can look at the data, find problems before they happen, and suggest ways to fix them.
For example, AI can predict errors based on past data and help you prevent them. It can also find ways to make your processes run smoother and faster. If something goes wrong, it can quickly point it out so you can fix it right away.
AI also helps make sure your work can be repeated. It can show you why something worked well, so you can do it again. This opens the gate for efficient, continuous improvement.
What is GxP OS?
Imagine a life sciences company bogged down by fragmented systems. Data is scattered across LIMS, ELN, EBR, MES, and countless spreadsheets. The result? Inefficiencies, errors, and a constant struggle to maintain compliance.
Now, imagine a scenario where quality management isn’t a cumbersome, reactive process but a streamlined, proactive approach that enhances every aspect of operations. GxP OS makes this future a reality by providing the tools and capabilities needed to ensure that quality is always at the forefront. With advanced integration capabilities, real-time data analysis, and alignment with Quality by Design (QbD) principles, GxP OS sets a new standard for quality management in the life sciences industry.
GxP OS unifies all procedures into a single, integrated system. Every procedure is version-controlled, making sure that the latest practices are always in use without manual updates. This level of control not only improves accuracy but also enhances compliance, making audits smoother.
This unified approach to quality management is transformative. By embedding quality into core operations, teams can work more swiftly and efficiently. Regular, incremental updates foster continuous improvement, allowing for early identification and resolution of issues. This proactive stance reduces the risk of major flaws and ensures that the entire organisation remains aligned with the latest standards and best practices.
But GxP OS doesn’t just unify procedure management and execution. It also integrates Internet of Things (IoT) devices, merging instrument data with offline data for analysis. This integration improves decision-making, efficiency, and quality control. Imagine having real-time data from laboratory instruments directly feeding into your quality management system. Anomalies or deviations can be detected and addressed immediately, significantly reducing the risk of errors and improving overall product quality.
How GxP OS is redefining quality management
The traditional QMS model can no longer keep up with today’s fast-paced, technology-driven environment. Traditional systems are too rigid and slow to adapt to changes.
In contrast, GxP OS is designed to be flexible and responsive, able to adapt quickly to new requirements and challenges. This agility issue, in particular, is crucial in an industry where regulations and standards are often evolving. By keeping pace with these changes, companies can make sure that they remain both compliant and competitive.
Enhanced integration and collaboration
GxP OS’s strength lies in its ability to integrate with various systems and tools seamlessly. Whether it’s LIMS, ELN, EBR, or MES, GxP OS acts as a central hub that consolidates all data and processes. This eliminates the silos and promotes a more collaborative environment. Teams can access and share data effortlessly, enhancing collaboration and driving more informed decision-making.
Real-time data and predictive analytics
One of the most significant advantages of GxP OS is its capability to use real-time data and predictive analytics. By continuously monitoring processes and collecting data from various sources, GxP OS can provide insights into potential issues before they escalate. Predictive analytics help in anticipating problems, optimising processes, and check that corrective actions are timely and effective.
Put regulatory on autopilot
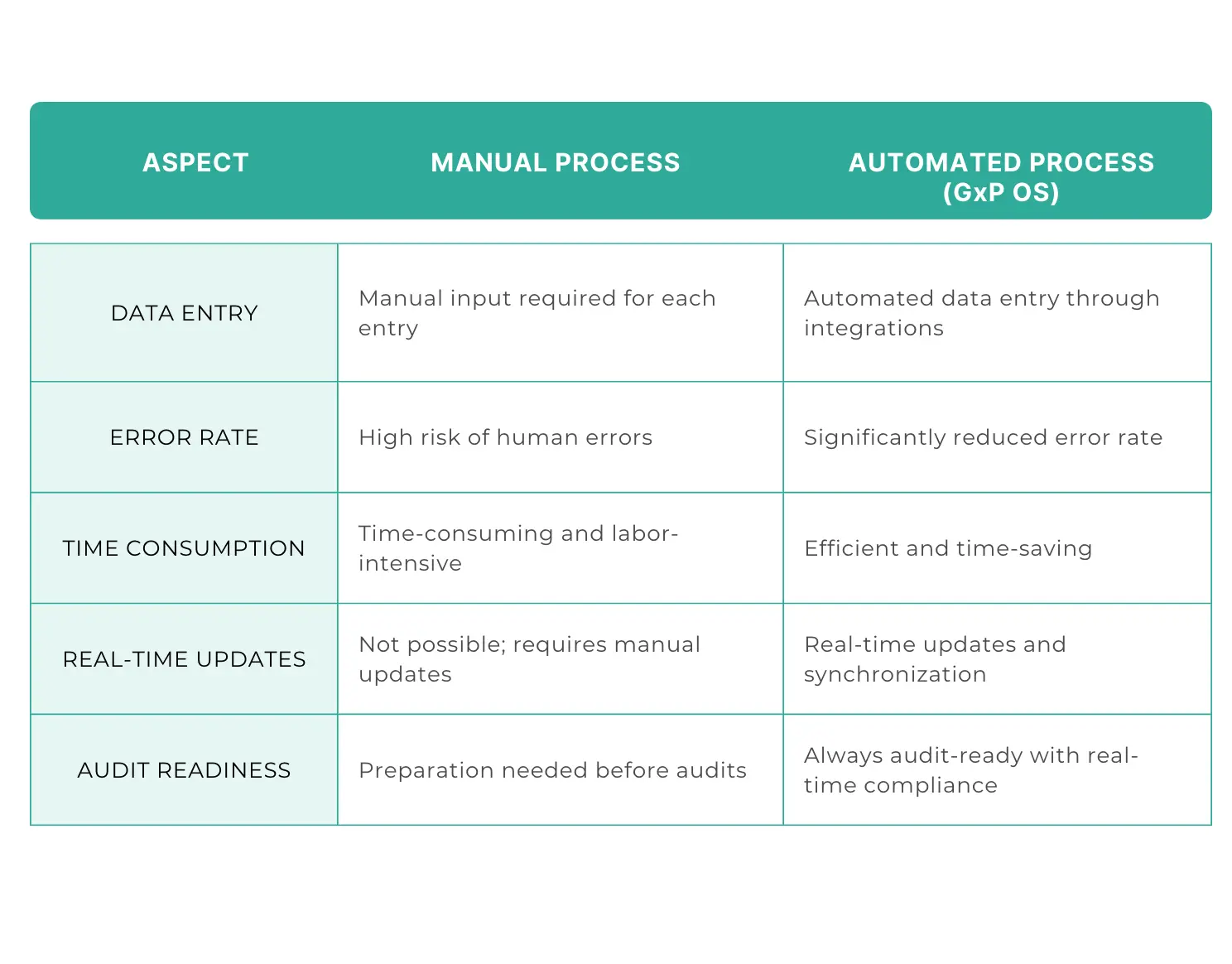
Compliance with regulatory standards is non-negotiable in the life sciences industry. GxP OS ensures that all processes and documentation are in line with the latest regulations. Its real-time monitoring and reporting features make it easier to maintain compliance and prepare for audits. With GxP OS, companies can have confidence that they are always audit-ready, reducing the stress and resource strain typically associated with compliance activities.
Becoming agile
GxP OS promotes a culture of continuous improvement. By providing tools for regular updates and process optimisation, it helps organisations stay ahead of the curve. This focus on continuous improvement fosters innovation and allows companies to adapt swiftly to new challenges and opportunities.

