Quality and compliance in manufacturing are essential. Good Manufacturing Practice (GMP) is the standard that helps achieve this. GMP guidelines help make sure products are consistently produced and controlled according to standards, by having detailed documentation, training, and testing.
This article explains what GMP software is, its benefits, and key features. We'll explain different types of GMP software and industry applications. There's also a comparison of the top GMP software templates to guide you in choosing the best fit for your needs.
GMP Software
What is Good Manufacturing Practice (GMP)?
Good Manufacturing Practice (GMP) is a system for making sure that products are consistently produced & controlled, following quality standards. It's intended to reduce the risks involved in any manufacturing process that cannot be removed by testing the final product. The main features of GMP include detailed documentation of processes, following high hygiene standards, good team training, and consistent testing of products to match specifications.
GMP guidelines don't offer specific instructions on how to manufacture products. Rather, they're general principles that should be considered during production. It's a manufacturer's responsibility to choose the best quality processes for its needs.
What is GMP software?
Definition
GMP software refers to a suite of software tools intended to help manufacturers in comply with good manufacturing practices. As we explained above, these practices are regulatory requirements that manufacturers need to follow to make sure their products are made properly.
There are different types of software under this umbrella term which we'll explore in more detail later on. Generally speaking, GMP software is a tool for manufacturers to remain compliant by digitally managing documentation, processes, and quality control.
Types of GMP software
The main types of GMP software are:
Electronic Batch Records (EBRs)
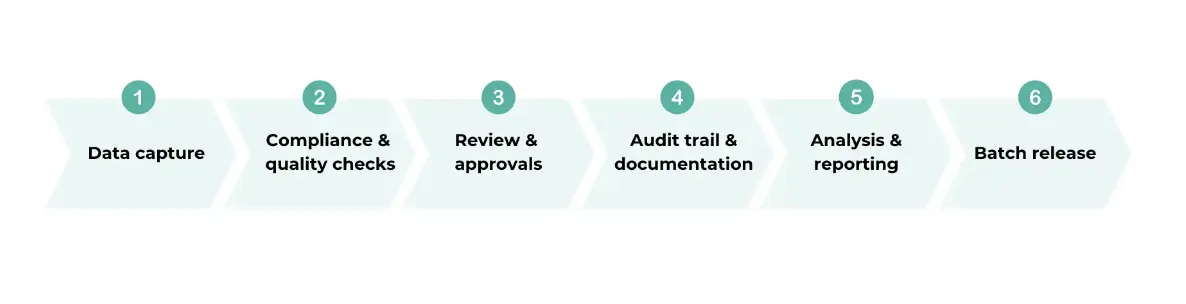
EBRs are detailed documents that track every step of making a product in manufacturing plants. These files include what materials are used, the unique ID for each product batch, how long each part of the process takes, and the checks done to ensure the product is made correctly. BMRs also note when things don't go according to plan and what actions are taken to fix those issues. This kind of attention to detail helps make sure that every product is consistent in quality and safe for use.
If you're looking for more detail about this topic, you can read these blog posts:
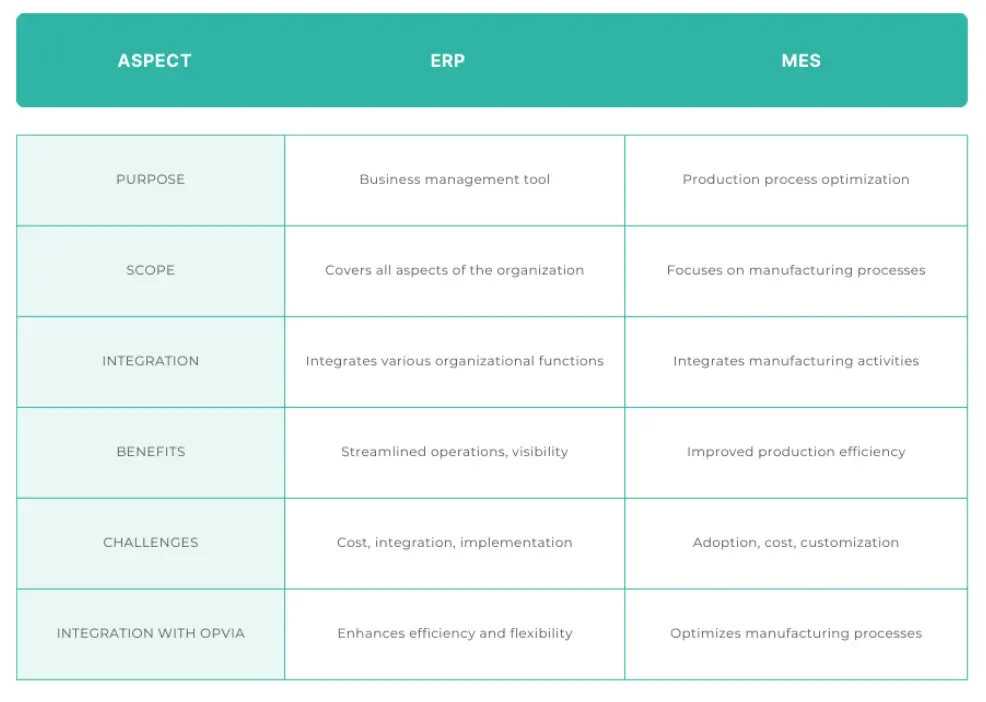
Manufacturing Execution Systems (MES)
A Manufacturing Execution System (MES) is a system used in manufacturing industries to track, record, and document the manufacturing process from raw materials to the final finished product. A MES integrates all manufacturing processes and activities, from the scheduling, planning, manufacturing data, quality control, inventory management, and can even include aspects such as approvals and reporting. The MES act as a centralised platform for communication and collaboration between various departments and to both internal and external stakeholders involved in the manufacturing process.
Again, we've written in more detail about MES here:
There are also more general features and tools that come under the term GMP software. Though they might not strictly be a “solution” in-of-themselves like MES or EBR, they often are features within these templates. Of course, this depends on the software vendor, what features they offer and what they choose to call their software.
In any case, here are a couple of these common features:
Quality control (QA) and quality assurance (QA)
This refers to tools for real-time monitoring of production quality, automated quality checks, & integration with other tools like laboratory information management systems (LIMS) to make sure that the product specifications are matched.
Compliance & audit management
These kinds of features help control audits & inspections. This may include scheduling, tracking, as well as different reporting tools that help prepare for and follow-up on regulatory audits.
Risk & change management
Features like this are intended to assess & document risks involved with a product's quality and compliance.
What industries is GMP software used in?
- Pharmaceuticals
- Biotechnology
- CDMO
- Food and Beverage
- Cosmetics
- Medical Devices
Benefits of using GMP software
Using Good Manufacturing Practice (GMP) software provides several benefits across different steps of manufacturing operations.
Greater compliance
Compliance with regulatory standards is the main use of GMP software. It's intended to follow the strict guidelines set by authorities like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency), making sure that companies can pass audits and avoid the costly impacts of non-compliance. Non-compliance can mean fines, costly product recalls, & damage to reputation.
So how does GMP software help with compliance?
- Automated documentation: For a start, GMP software automates the creation, storage, and management of documents like SOPs (Standard Operating Procedures) and EBRs (Electronic Batch Records). This means that documents are always up-to-date and ready to be inspected.
Helpful resource: You can use this SOP Template to document your procedures if you don't have any kind of GMP software.
- Audit trail: The software also provides detailed and time-stamped audit trails for every action taken during the manufacturing process. This makes it easier to trace issues or deviations back to their source of the problem.
- Consistent procedures: GMP software helps make sure that protocols and procedures are followed to a T, which reduces risk of human error.
More efficient operations
GMP software significantly enhances operational efficiency by streamlining processes and reducing the need for manual interventions, which can be time-consuming and prone to error.
- Process optimisation e.g. reducing waste & downtime
- Scalability
- Faster decision making
Better data management
Good data management is crucial for all manufacturing operations, and GMP software is great at organising, storing, and then analysing lots of data related to manufacturing.
Centralised repository of data
One of the main benefits of GMP software is that you get a single, centralised repository for all compliance and production-related data. This simplifies management and makes information more accessible. A byproduct of this is that you also get better collaboration between departments.
Advanced analytics
GMP systems usually come with advanced analytics features, allowing manufacturers to extrapolate actionable insights from data.
Key features to look for in GMP software
When choosing GMP software, it's important to focus on a set of core features that will help you remain compliant, improve operations, and offer efficient data management abilities.
Here's a rundown of the must-have features to look for in GMP software:
Audit trails
An audit trail is a feature that records every action taken within the software system. It provides a log of exactly when an action was taken and who it was taken by.
Audit trails enable the following features:
- Time-stamped entries
- Immutability ie. audit trails cannot be altered after the fact, maintaining the integrity of the logs for compliance.
- Ease of access
Data integrity controls
Data integrity is crucial in GMP software to make sure that data, once entered into the system, stays accurate and reliable throughout its lifecycle.
Data integrity controls include:
- Validation data ie. a validation mechanisms makes sure that all data entered meets predefined criteria to reduce errors.
- Access controls
Real-time monitoring
Being able to have visibility over the manufacturing processes at all times helps to easily find and address issues as they're happening.
GMP software does this in a few different ways:
- Dashboards: dashboards offer a real-time overview of metrics and KPIs, to get immediate insights into operations.
- Alerts & notifications: automated updates are sent in response to any events or deviations from standard operating procedures, enabling quick corrective actions.
- Integration with production equipment: data from equipment is automatically collected and analysed.
User-friendly interface
Friendly UI not only makes usability easier but also means that training teams and getting them to adopt the software becomes a lot easier. Whatever EBR, MES or any other system that is chosen should have intuitive navigation that is clear and helps users to easily find information.
Scalability
Having a system that can grow with your business without the need for a complete software overhaul is important. There's nothing worse than a system that starts to lag in performance as more data is fed into it. Or if your company needs to adopt an eQMS, LIMS, or ELN in addition to GMP software, your existing provider should be able to supply that. An incomplete software that forces you to have to stitch together lots of third party templates is a nightmare for having visibility over the product life cycle.
So, in terms of scalability, it's important to consider whether your GMP software provider offers:
- Modular design: a design that allows additional features & functionalities to be added as and when, without disrupting existing operations.
- Cloud-based system: a system that offers flexibility, easy scalability, and reduced IT maintenance costs compared to on-premise templates.
Comparison of top GMP Software products
We've written more extensively about this topic in another article titled EBR system system comparison: Top 5 templates for 2024. But to cut a long story short, here are some of the best templates providers.
- MasterControl
MasterControl offers comprehensive features that support all aspects of GMP compliance.
Strengths:
- Strong in document control, process management, and compliance tracking.
- Offers templates across quality and manufacturing
Weaknesses:
- Can be complex to implement,
- Costly for smaller setups
- Veeva Systems
Veeva offers cloud-based templates for life sciences industries, offering end-to-end templates.
Strengths:
- Makes complex and extensive processes easier to manage
- Highly configurable
- Cloud-based
Weaknesses:
- High depending on other Veeva systems which could be a limitation if there is a need to integrate with other third party systems.
- Seal Platform
seal's cloud platform simplifies how organisations manage and execute GxP procedures, integrating every step and data point across the product lifecycle. By digitising procedures, the platform automates compliance and data workflows, making it easier to maintain standards and efficiency.
Strengths:
- Highly intuitive interface
- Customisable templates
- End-to-end platform that can also integrate with any third party software
- Extensive support and training
Weaknesses:
- Tailored setup requires initial planning to ensure optimal fit with specific manufacturing goals.

