An electronic quality management system (eQMS) digitises how quality control is managed. It replaces paper-based systems with digital systems, integrating record-keeping, workflow automation, and quality checks into one platform.
One of the key features of an eQMS is its database which simplifies tasks like document tracking and audit management, whilst making sure that standards like ISO and FDA regulations are being met. These systems enable manufacturers to better handle quality issues, corrective actions, and improve product standards. Equipped with real-time analytics, an eQMS can also help companies cut waste and refine their processes.
This article will break down the basics of eQMS, explaining how they work, their benefits, and their importance in product manufacturing.
Limitations of paper and spreadsheets
Many companies still rely on manual or paper-based record-keeping to track quality controls, a process that is very time-consuming and prone to errors. These paper-based systems require extensive physical storage, make it challenging to retrieve specific information quickly, and are just inefficient.
Many labs and manufacturers still struggle with cluttered paperwork…
Reliance on paper for record-keeping results in serious limitations. Paper records are not just susceptible to physical damage like water, crumpling, or just being misplaced but also are incredibly inefficient for organising large amounts of information or remaining compliant. Likewise, updating these paper records is slow-moving, resulting in outdated or conflicting versions of documents.
… Or risk accuracy mistakes with overwhelming spreadsheets
While spreadsheets are a step up from paper in terms of digitisation, they come with their own set of problems. First off, they lack real-time collaboration features, leading to multiple versions of the same document and confusion over which one is the most up-to-date. They're also prone to human error: a simple typo can have far-reaching consequences, skewing data analysis & leading to inaccurate results. Spreadsheets are also limited in their ability to manage complex relationships between data points, making them unsuitable for tracking intricate quality control processes across multiple products or locations.
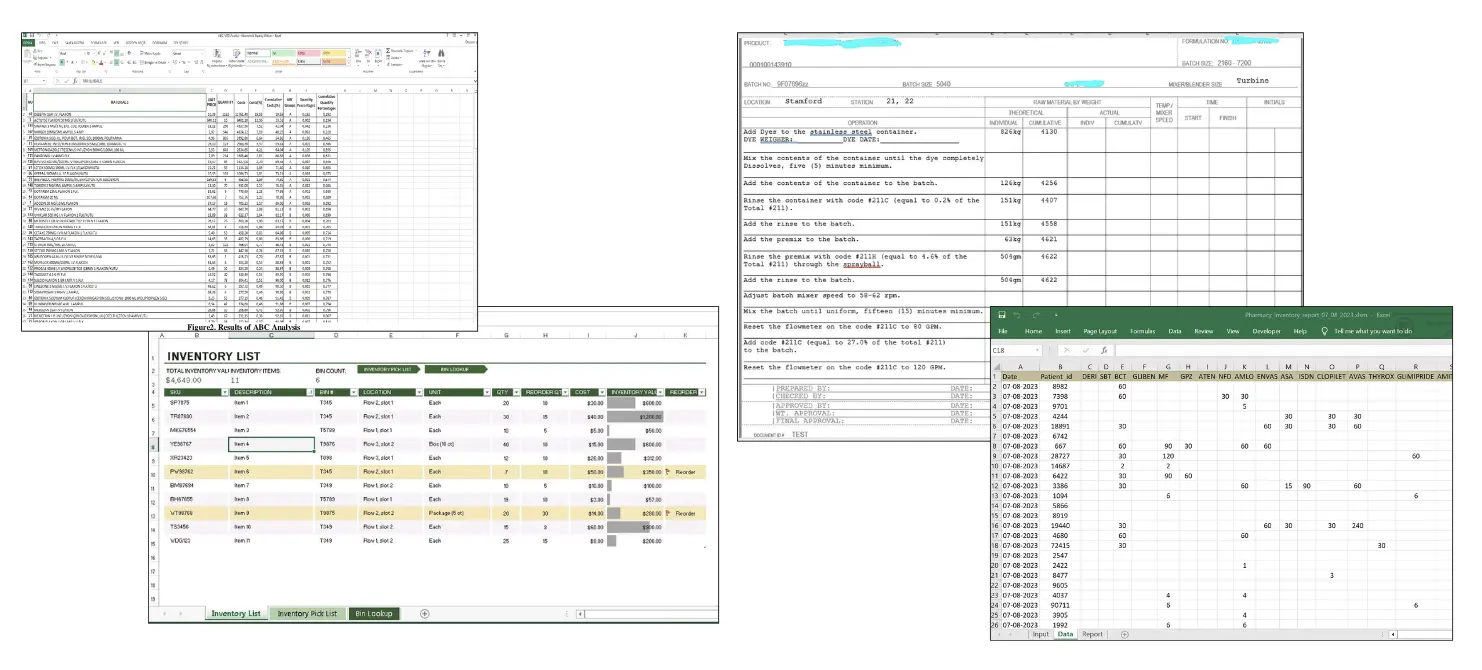
What is an eQMS?
An electronic quality management system (eQMS) digitises quality control processes, replacing paper and spreadsheets. It integrates record-keeping, workflow automation, and quality checks into a single digital platform.
A key feature of an eQMS is its database, which enables document tracking, audit management, and compliance with standards like ISO and FDA regulations. An eQMS system helps manufacturers address quality issues, manage corrective actions, and monitor product standards.
An eQMS offers a cleaner and more accessible way of overseeing quality management, from document control and training to audits and supplier management. Not only does this improve efficiency but also enables greater accuracy and traceability. Manufacturers can instantly access and analyse their data, identify trends, and conduct corrective actions more quickly.
QMS v. eQMS
A quality management system (QMS) is a set of procedures and guidelines a manufacturer follows to ensure its products and services meet quality standards & customer expectations. It's a structured way of doing things that shows how to make products the right way, making sure customers are satisfied and everything complies with laws and regulations.
An electronic quality management system (eQMS) is the digital version of a QMS. It takes all those guidelines and procedures and puts them into a software solution. This makes it easier to manage because everything is in one place, accessible from anywhere, and it reduces paperwork. The big difference is that an eQMS uses technology to streamline and improve quality control processes. The distinction can be useful to understand, but often when someone refers to a QMS they are actually referring to an eQMS. So the terms may be used interchangeably.
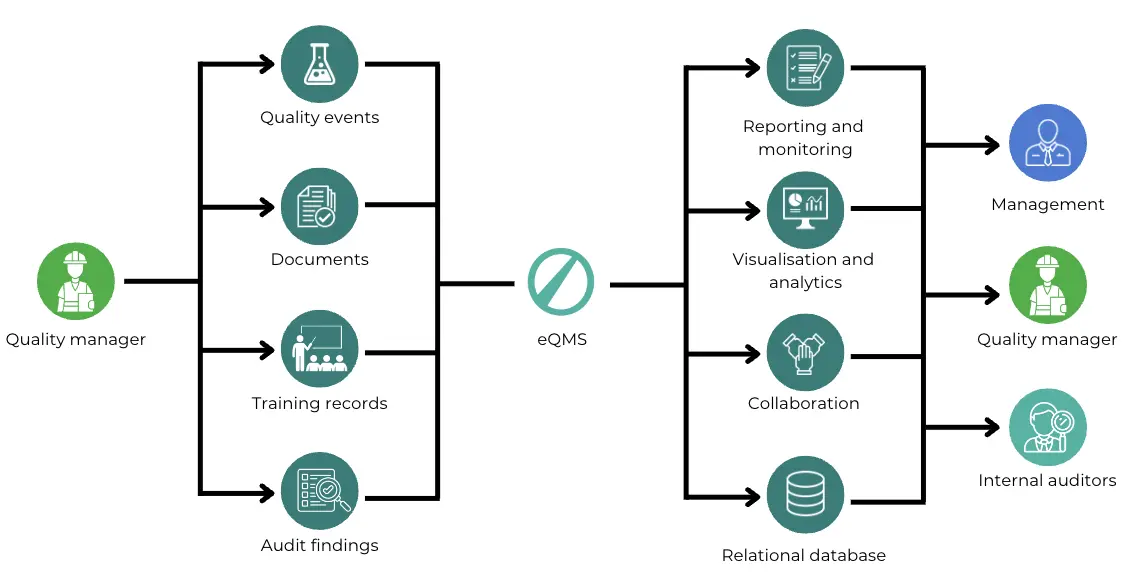
Key features of an eQMS
What's included under the term eQMS can be quite varied. For example, sometimes an eQMS can also include features that are more commonly associated with Electronic Batch Records.
Generally speaking though, common features of an eQMS include:
- Document management
- Training management
- CAPA (Corrective and Preventive Actions)
- Audit management
- Risk management
However, these are really just the most basic features that you can expect with an eQMS. Top-tier providers should offer more widespread and comprehensive capabilities like:
- Version control
- Automated workflows
- Advanced analytics & reporting
- Integrations with other tools
- Complaints management
- Inventory and supplier quality management
Benefits of implementing an eQMS
From a high-level view, an eQMS can be useful in improving how enterprises manage quality, compliance, and product development.
One such benefit is real-time data access. In regulated industries, like the pharmaceutical industry, real-time data access allows for immediate visibility into the production process. For instance, if a batch of medication shows unexpected results during quality checks, an eQMS instantly alerts the quality team. They can then quickly investigate and implement changes or halt production if necessary, preventing both potential health risks and financial loss. The immediacy of this kind of oversight makes sure that every decision is informed by live data.
An eQMS can also automate the tracking and management of compliance documents, like safety standards and allergen controls. For food manufacturers, for example, regulatory compliance is a must. So when a food safety inspector requests documentation during an audit, the manufacturer can instantly provide comprehensive records, showing transparent compliance with regulations like the FDA's Food Safety Modernisation Act. This not only makes the audit process easier to manage but also greatly reduces the risk of violations and recalls.
In terms of product development, an eQMS can significantly improve the efficiency of processes. Companies like medical device manufacturers are highly regulated, requiring rigorous testing and documentation to make sure their products are safe for patient use. An eQMS enables a company to have a centralised platform where design, engineering, and quality teams can collaborate efficiently rather than the different stages of the product life-cycle being siloed.
For instance, if a prototype device fails to meet the required biocompatibility standards during testing, the test results and feedback are immediately available within the system. Engineers and designers can then access this information, understand the shortcomings, and make changes to the design. Again, this kind of direct and real-time procedure can accelerate the development process, whilst also ensuring compliance with regulatory standards like those set by the FDA or EMA. It's no surprise then that MedTech companies that use eQMS have shown a reduction in the time it takes to bring new, safe medical devices to market.
Selecting an eQMS
Not all eQMS templates are created equal. Many suffer from poor user interface designs that are not intuitive, making them difficult to use and leading to low adoption rates within an organisation. Others may be overly rigid, offering little to no customisation, which means they can't adapt to the specific workflows or regulatory requirements of different industries. Many eQMS templates struggle with basic integration issues, unable to connect with other critical software tools within an organisation, which ultimately defeats the purpose of an eQMS with siloed data and more inefficiency.
Selecting the right EQMS software also requires careful consideration to ensure it aligns with an organisation's unique needs. When evaluating templates, focus on the specific features that support your enterprise quality management goals. An eQMS with robust document management and CAPA features is essential for maintaining high compliance & quality standards.
Scalability is another factor. As your business grows, your eQMS should easily adapt to this growth, accommodating more users, processes, and data without compromising performance. Many startups or small businesses start with a small team and a handful of processes but often need to scale up their production and team as they grow. Your eQMS software must support this growth seamlessly.
Integration capabilities are equally important. The best eQMS software should smoothly integrate with other systems you already use, such as ERP (enterprise resource planning) or CRM (customer relationship management) platforms. Data needs to be able to flow freely between different systems otherwise it becomes siloed and impossible to work with.
Compliance with FDA CFR Part 11
For labs or manufacturers looking to adopt an eQMS, picking the right one can be tricky. A key thing to keep in mind for any eQMS buyer, especially in life sciences or other regulated industries, is the system's compliance with FDA 21 CFR Part 11 regulations (for the USA).
This rule governs the use of electronic records and electronic signatures, setting standards for their acceptable equivalent to paper records and handwritten signatures. It's important to be sure that the system you use for managing your digital records is both appropriate and legal. Using a makeshift or outdated system that doesn't meet these standards, for example, could end up in non-compliance issues, risking failed audits, and large fines.
Other regions have similar guidelines and standards to the US's 21 CFR Part 11. The European Union's Annex 11 guidelines and global standards like ISO 27001 offer equivalent regulations for managing electronic records and signatures.
When looking into eQMS that is compliant with these standards, key aspects buyers should focus on include:
- Security and access controls
- Electronic signatures
- Audit trail capabilities
- Data integrity and reliability

