Document control is a key part of quality management, ensuring that all documents are accurate, up-to-date, and accessible. This article will cover how document control involves creating, reviewing, approving, distributing, and archiving documents to maintain consistency and compliance. This is crucial in industries like pharmaceuticals, food and beverage, and manufacturing, where strict documentation ensures product safety and meets standards. We'll explore guidelines from ISO 9001, Good Documentation Practices (GDocP), and Good Manufacturing Practices (GMP). ISO 9001 outlines quality management system requirements, GDocP ensures documents are clear and accurate, and GMP guarantees product quality.
What is Document Control?
Document control is the process of managing and organising documents so that everyone in an organisation is working with the most up-to-date information. It involves creating, reviewing, approving, distributing, and archiving documents in a way that keeps everything accurate and consistent. This process includes keeping track of changes and revisions, controlling who can access the documents, and making sure outdated documents are removed.
Key Standards in Document Control
There are some key standards for document control and how it should be handled. These include ISO 9001, Good Documentation Practices (GDocP), and Good Manufacturing Practices (GMP).
ISO 9001
ISO 9001 is a standard focused on quality management and customer satisfaction. For document control, it requires having a quality manual with documented procedures. Documents need to be approved before use, regularly reviewed, updated, and easily accessible. It's also important to track any changes and know why they were made.
Good Documentation Practices (GDocP)
Good Documentation Practices (GDocP) provide guidelines for making and managing documents in regulated industries. GDocP makes sure that documents are complete, accurate, and easy to understand. All documents should follow the same format and style for consistency. Keeping track of who created, reviewed, and approved documents helps with traceability. Plus, documents should always be easy to read.
Good Manufacturing Practices (GMP) Documentation
GMP documentation is important in industries like pharmaceuticals, food and beverages, and medical devices. It makes sure products are made and controlled to high standards. This includes Standard Operating Procedures (SOPs) with detailed task instructions and batch records for each product batch to ensure traceability. Training records are also important to show that employees are properly trained for their tasks.
Document Control Procedures
Effective document control procedures make sure that all documents are accurate, up-to-date, and accessible to those who need them. To give you an idea of how this looks like in practice, ere’s a step-by-step guide on creating and maintaining controlled documents.
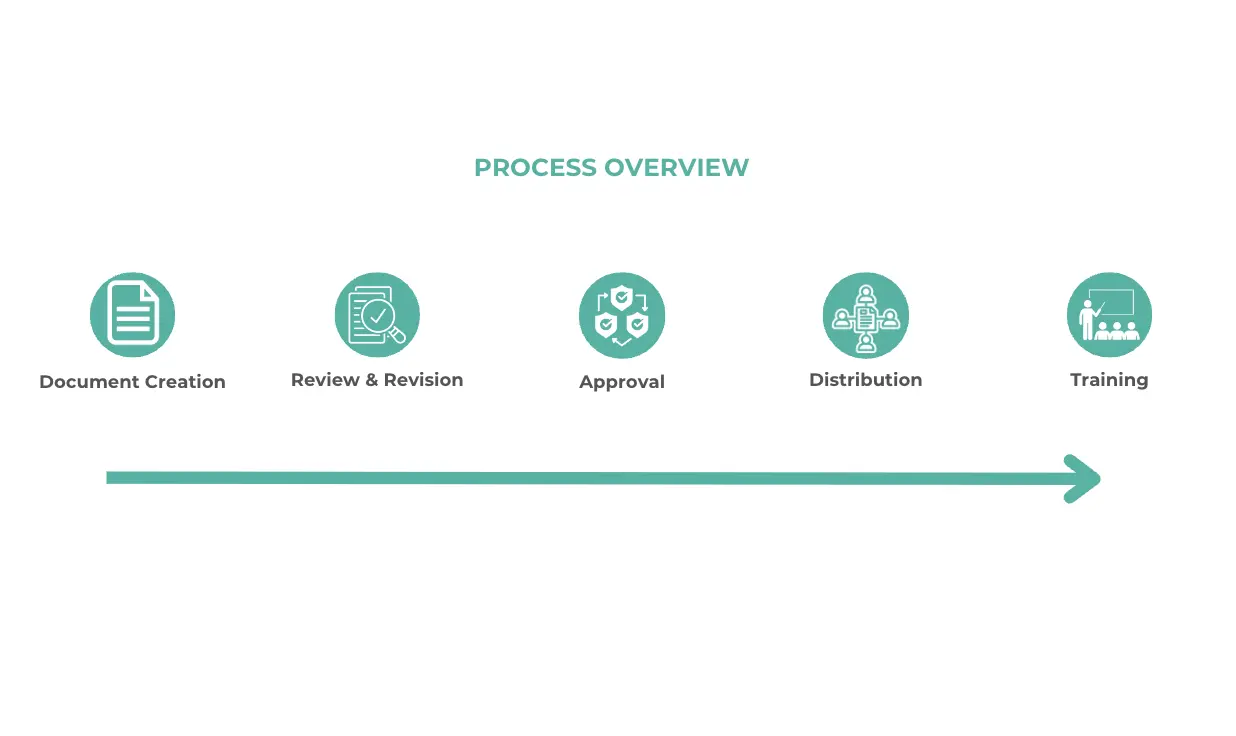
Step-by-Step Guide on creating and maintaining controlled documents
- Document Creation: Start by drafting the document. Make sure it has all the necessary information, written clearly and concisely. Use a standardised template to maintain consistency across all documents.
- Review and Revision: Once the initial draft is complete, circulate it for review. Collect feedback and make necessary revisions.
- Approval: After revisions, the document needs to be approved. This involves getting sign-offs from relevant stakeholders, like department heads or quality managers.
- Distribution: Once approved, the document should be distributed to all relevant team members. Check that it’s easily accessible, whether through a centralised document management system or another method.
- Training: If the document introduces new procedures or changes existing ones, you should provide training to make sure everyone understands and can adopt those changes.
Approval Workflows and the Function of QA
The role of quality assurance (QA) is pretty important throughout this process. They make sure that all documents meet quality standards and regulatory requirements, maintain consistency across all documentation, and verify that all changes are properly documented and traceable.
Importance of Version Control and Archiving
Version control is the process of tracking all changes made to a document, including who made the changes and why. This prevents confusion over document versions and makes sure that updates are properly recorded. Some of the key practices for this include, assigning version numbers to each revised document, keeping a log of changes, and limiting editing rights to authorised personnel.
Archiving involves storing older document versions for easy retrieval when needed, providing a historical record of all changes for audits and compliance. Here, best practices include using a centralised system for storing archived documents, including metadata like version number, date, and author for easy retrieval, and also setting clear retention policies to know how long documents are kept.
Function of Document Control Software
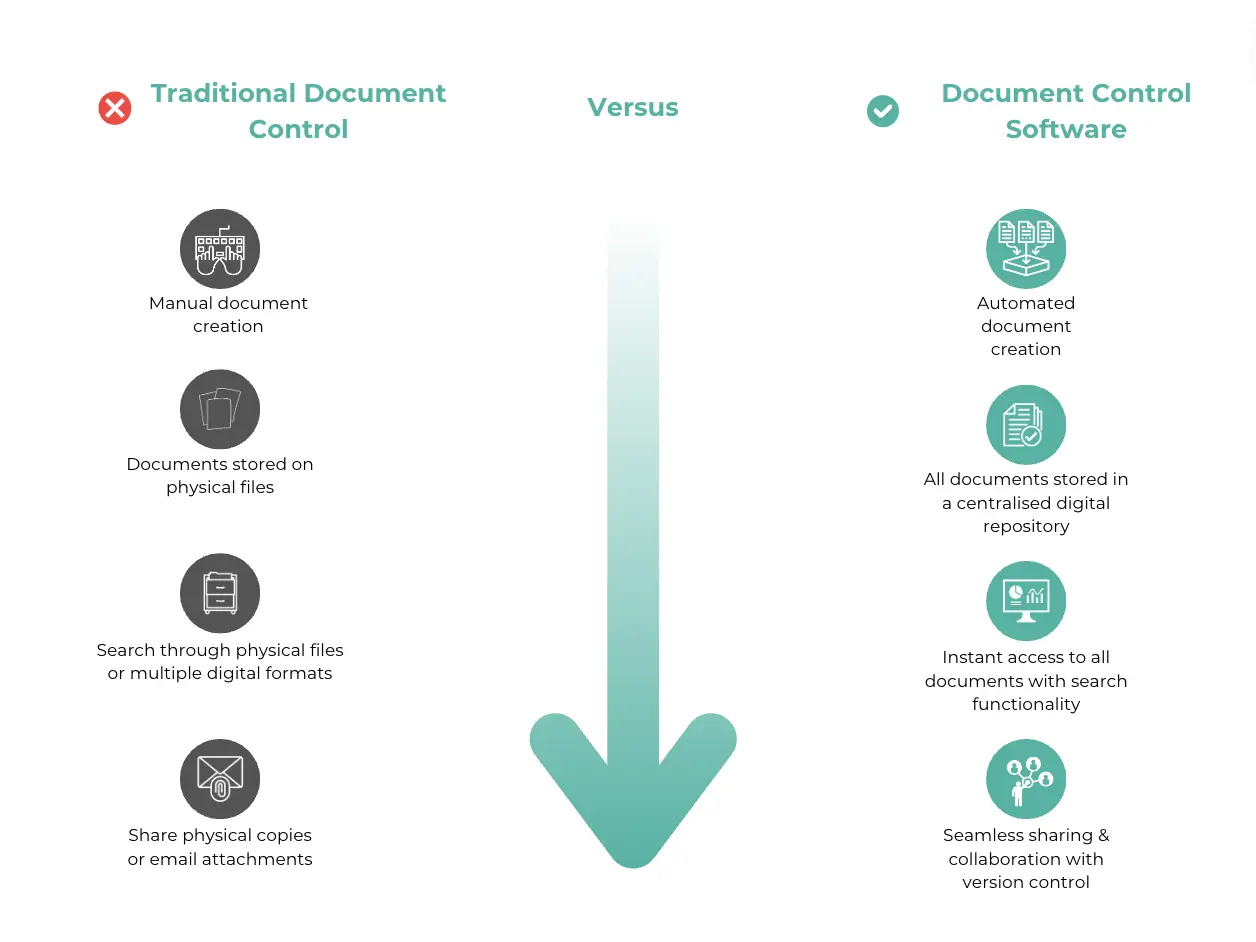
Using document control software can make managing documents much easier and more efficient. This kind of software, like seal’s Free eQMS, helps keep everything organised and makes sure that documents are accurate, up-to-date, and easily accessible.
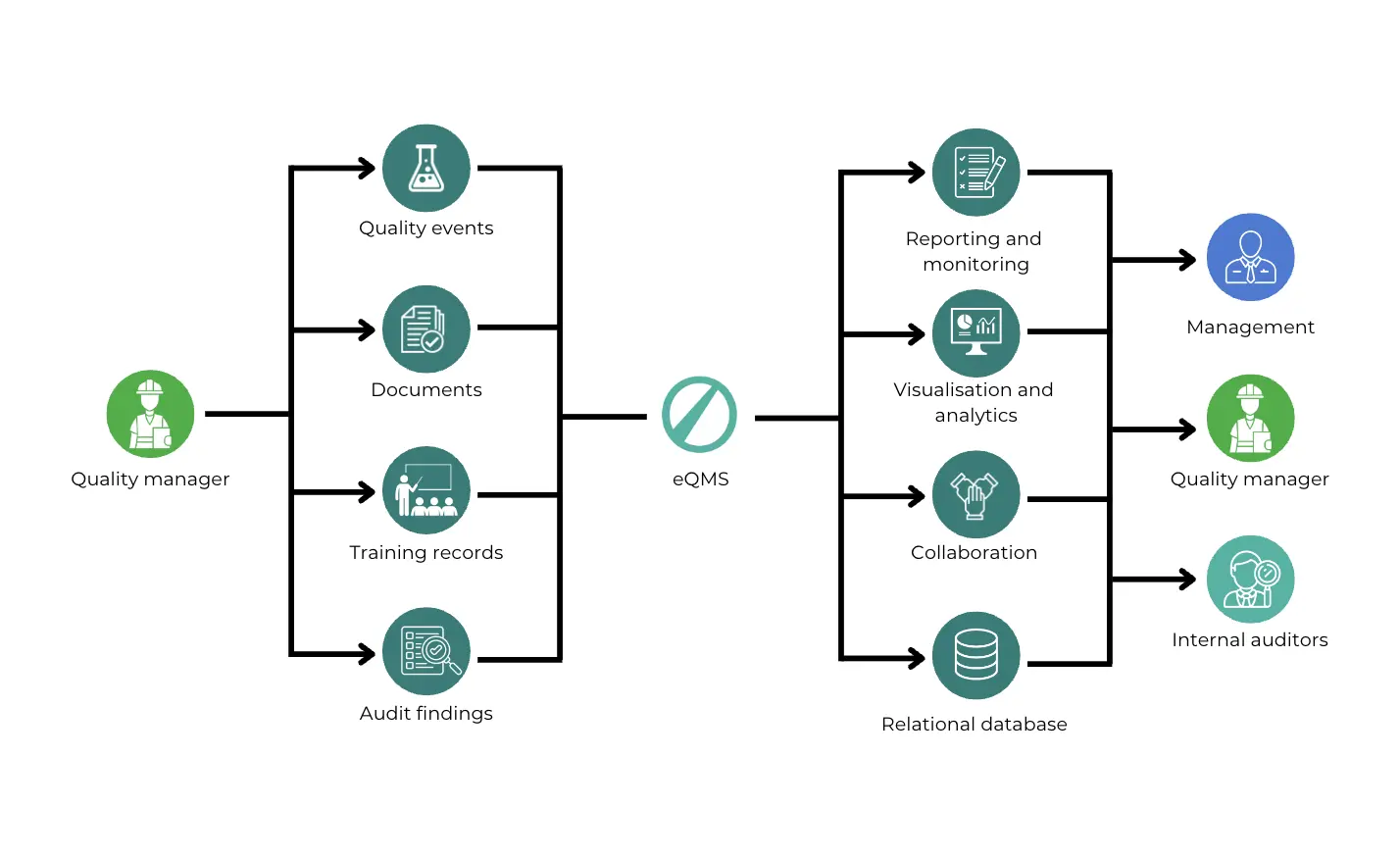
Benefits of using document control software
One of the biggest advantages of document control software is automated version control. This feature makes sure that everyone is always using the latest version of a document by automatically tracking changes and keeping a detailed revision history.
Access control is another important feature, as it restricts document access to authorised personnel only, reducing the risk of unauthorised changes and ensuring that document integrity is maintained.
Audit trails help with transparency and traceability. Document control software logs all document activities, showing who made changes, what changes were made, and when. This is particularly useful during audits. Workflow automation streamlines the approval process by routing documents to the right people, sending notifications, and tracking progress.
There are a lot of benefits to using document control software, so here’s a brief list of some the key benefits:
- Automated Version Control: Always have the latest version.
- Access Control: Restrict access to authorised personnel.
- Audit Trails: Maintain detailed logs of document activities.
- Workflow Automation: Streamline the approval process.
- Centralised Storage: Simplify document retrieval.
How Software Can Support ISO 9001 Compliance
ISO 9001 has some strict requirements for document control, and using the right software can help meet these standards. For example, maintaining a quality manual that includes the scope of the QMS, documented procedures, and process descriptions becomes much easier. The software ensures documents are approved, reviewed, updated, and accessible as required. Detailed records of changes are kept, providing the necessary traceability.
Document control software improves efficiency by automating repetitive tasks, such as document routing and notifications, allowing employees to focus on more important activities. During audits, having all relevant documents and records easily accessible, along with comprehensive audit trails, simplifies the process and demonstrates compliance with ISO 9001 requirements.
FAQs on Document Control
What is a QA document controller?
A QA document controller manages quality assurance documents. They make sure that documents comply with regulations, are properly reviewed and approved, and are accessible to authorised personnel. They maintain the integrity of the quality management system.
What are the 4 tiers of quality documentation?
The four tiers of quality documentation are:
- Quality Manuals: Outlines the quality management system.
- Procedures: Detailed steps for specific tasks.
- Work Instructions: Specific instructions for consistent task performance.
- Records: Evidence that procedures and instructions have been followed.
What does it mean to QC a document?
To QC (quality control) a document means to review it for accuracy, completeness, and compliance with standards. This ensures the document meets quality criteria before approval and distribution.
What is QA QC documentation?
QA QC documentation includes all documents used in quality assurance and quality control processes. This includes quality manuals, procedures, work instructions, and records demonstrating compliance with quality standards and regulations. These documents ensure product and service consistency and quality.

