Understanding the differences between Quality Assurance (QA) and Quality Control (QC) is essential for effective quality management. While QA focuses on preventing defects through process improvements, QC concentrates on identifying and correcting defects in final products. This article explains what both QA and QC are in detail, the key practices used in both, examples of how they might be integrated into a process, and the key differences between them.
What is Quality Assurance (QA)?
Definition
Quality Assurance (QA) is a key part of quality management that focuses on preventing defects and making sure that products meet set standards. QA is a process-oriented approach to quality management. The aim of QA is to both improve and stabilise manufacturing processes to prevent issues before they ever even happen. By focusing on improving processes, QA helps manufacturers keep consistent quality across all stages of production.
QA involves setting up procedures that are intended to ensure that development and production processes are both efficient and compliant with industry standards. So, QA isn't just about improving product quality but also about increasing operational efficiency.
Key practices
Some of the most common QA practices are:
- Standard Operating Procedures (SOPs): SOPs are detailed, written instructions intended to make sure that processes are completed un a standardised way. They provide a clear framework for staff to follow so that every task is performed correctly and consistently.
- Audits: audits can be internal or external and are used to check that procedures (SOPs) are being followed correctly and that regulatory requirements are being met. Audits can also help find any areas for process improvement.
- Process documentation: records of procedures, workflows, and quality standards that standardise processes, assist in training, and ensure consistent quality control across an organisation.
What is Quality Control (QC)?
Definition
Quality Control (QC) focuses on making sure that final products meet the specified standards and requirements. QC is a product-oriented approach to quality management. The main goal of QC is to find and correct defects in the final products before they reach the customer.
As such, QC involves rigorous testing stages designed to detect any deviations from quality standards, so that only products that meet the required specifications ever meet the customer.
QC plays an important role in upholding a brand's reputation, meeting regulatory requirements, and making products safe.
Key practices
Some of the most common QC methods are:
- Product testing: this includes physical, chemical, and mechanical tests to check that a product performs as expected.
- Inspections: these can be visual, dimensional, or functional. Inspectors check for defects like surface flaws, incorrect dimensions, or functional failures.
- Statistical quality control (SQC): techniques like control charts, sampling plans, and process capability analysis are used to analyse production processes and spot areas of variation that may suggest defects.
Key differences between QA and QC
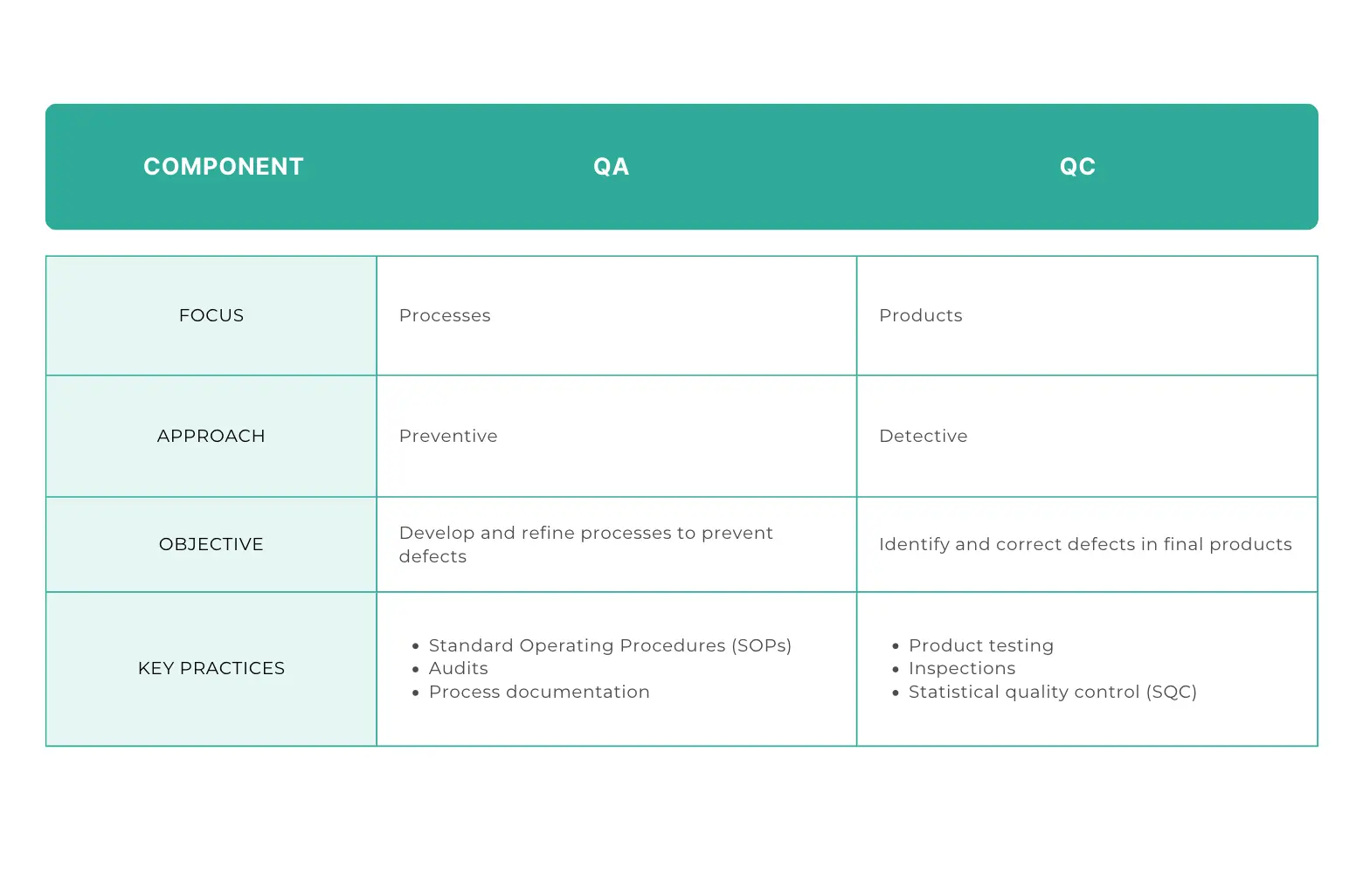
Process vs. Product
Quality Assurance (QA) and Quality Control (QC) have different but complementary roles in quality management.
QA is focused on processes. Its main objective is to create and refine processes involved in production and prevent defects from ever occurring in the first place. This is done by making sure that every step of the manufacturing process is well-designed and controlled.
In contrast, QC is focused on the products. The goal of QC is to find and then correct defects in the final products. QC activities is about inspecting and testing products so that they meet standards.
QA aims to prevent issues, while QC aims to detect and fix them.
Preventive vs. Detective
Another key difference between QA and QC is in the approach to managing quality.
QA is preventive. It tries to improve production processes to avoid defects. QA helps reduce the likelihood of errors.
But QC is reactive. It's focused on finding defects in the final products. QC involves testing and inspecting to find and correct issues that may have happened during the production process.
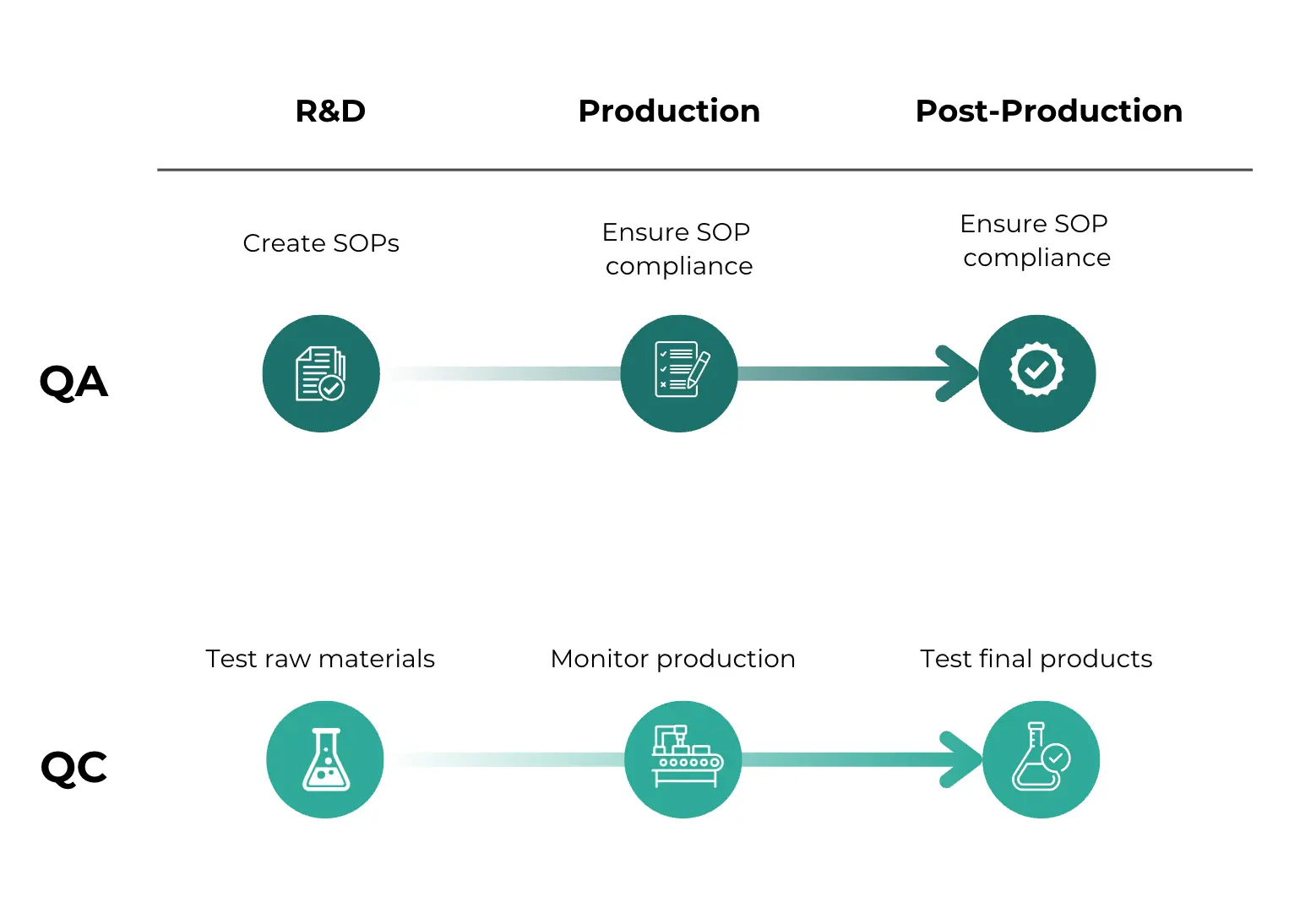
How QA and QC Work Together
Quality Assurance (QA) and Quality Control (QC) are complementary elements of a quality management system. Though they may focus on different aspects of quality, it's normal (and indeed essential!) to integrate them together into different parts of the product life cycle.
Let's take a look at an example of how they might fit into a pharmaceutical manufacturing process:
Development Phase
The QA team creates SOPs for drug formulation and offers training to the production staff. At the same time, the QC team tests raw materials like active pharmaceutical ingredients, to make sure they meet purity and potency specifications.
Manufacturing Phase
As production begins, QA ensures that all processes follow the established SOPs, overseeing steps like tablet compression and coating for consistency. The QC team monitors the production process, checking parameters like tablet weight, hardness, and dissolution rates to identify any deviations from quality standards.
Post-production Phase
After production, if the QC team finds any defects like inconsistent dissolution rates, they report these issues to QA. The QA team then investigates the root cause of the defects, updates the SOPs, and retrains staff to prevent recurrence. As you can see, there's a clear feedback loop between QA and QC which fosters continuous improvement. QA and QC efforts in conjunction help achieve greater efficiency, reduced waste, and product quality.