For labs looking to make the jump from laboratory notebooks to electronic laboratory notebooks (ELN), it can seem like quite a big change. Increasingly, it's becoming the norm to digitise lab records and procedures. This is particularly crucial for labs in highly regulated sectors like pharmaceuticals and biotech where data integrity, accurate results, and accessibility to research is so essential.
This article guides you through all the steps to smoothly transition to an ELN, with lots of specific tips to make things easier. This article covers:
- Defining success metrics
- Deciding on ELN requirements
- Organising existing notebooks
- Creating an implementation plan
- Training staff
Assess your needs and set clear goals
Transitioning to an Electronic Lab Notebook (ELN) is a big move for labs in industries like pharmaceuticals or biotech, where the accuracy, integrity, and accessibility of research data are so important. The first, and most obvious, step in transitioning to an ELN is to nail down exactly what your organisation's goals and requirements are.
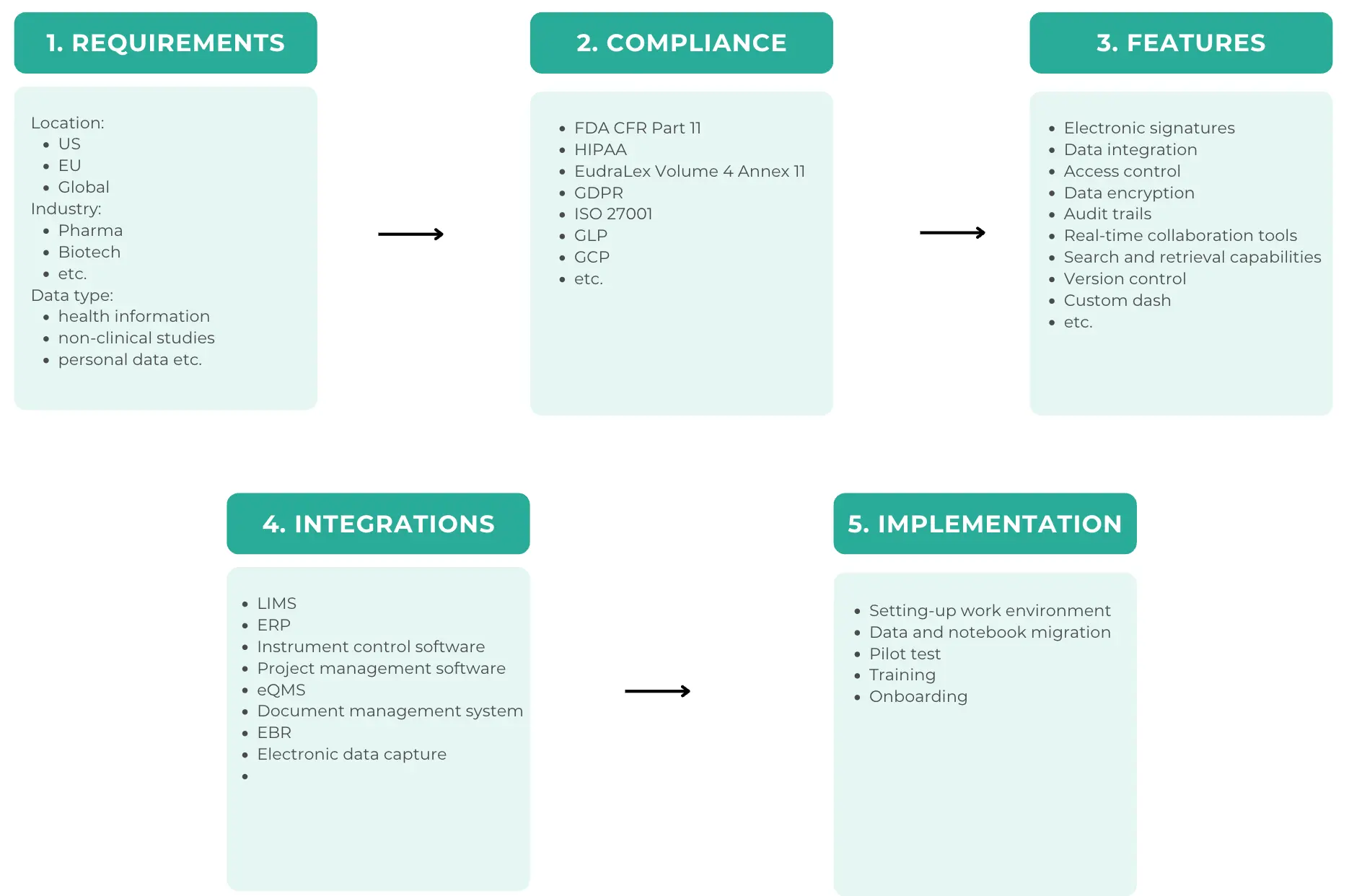
Identify functional requirements
We've actually written another article on this which you can find here, but to give you the gist of it here are the most important parts to be aware of.
- Regulatory compliance: For labs operating in highly regulated sectors, compliance with standards like FDA 21 CFR Part 11 in the United States, EudraLex Volume 4 Annex 11 in the European Union, and similar standards globally is a must.
An ELN needs to offer features that ensure data integrity, audit trails, electronic signatures, and time-stamped records.
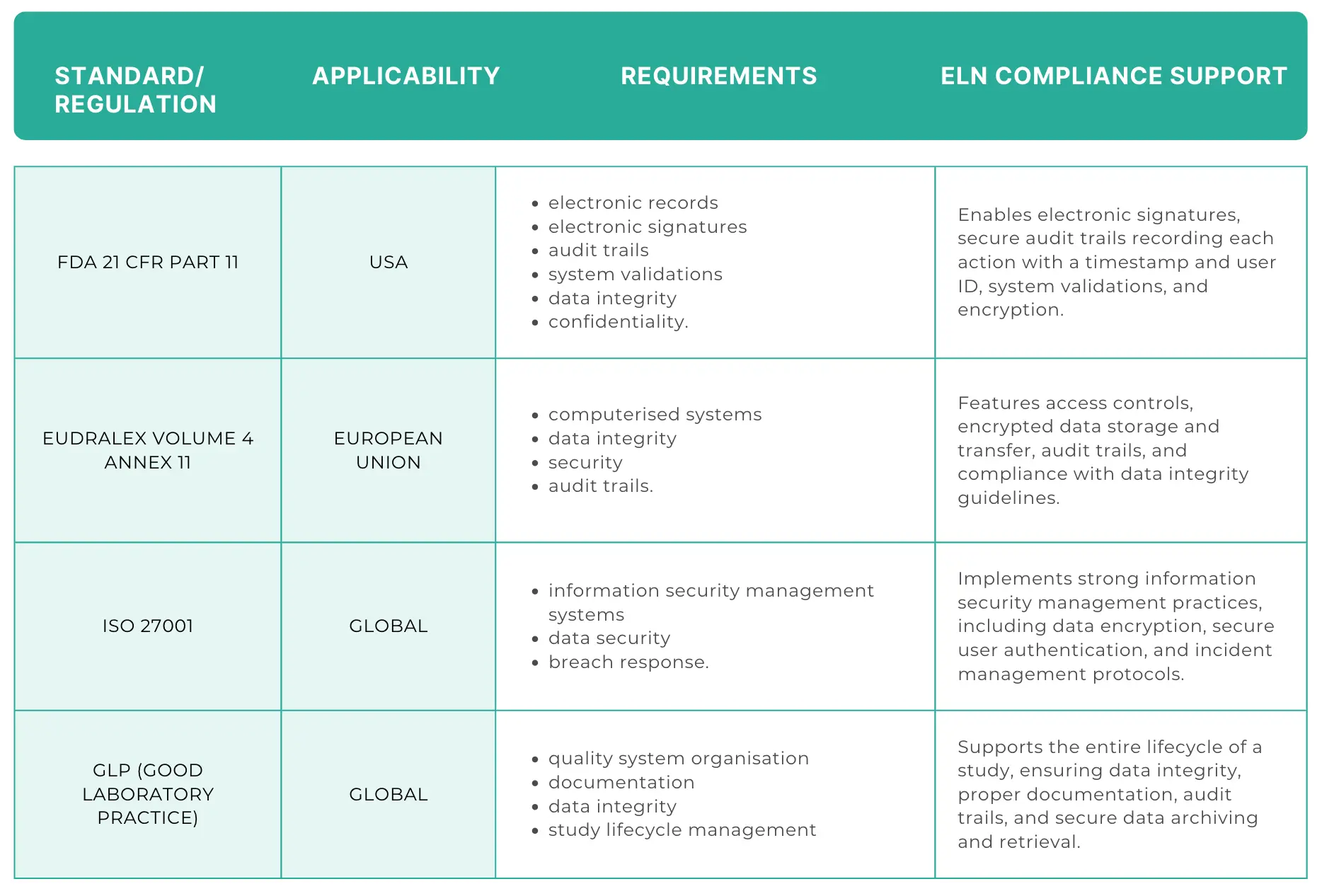
Pro Tip: use a compliance checklist tailored to pharmaceutical or biotechnology research when evaluating ELN vendors. This checklist should cover aspects like data encryption, access control, and audit capabilities.
- Integration with other laboratory systems: Seamless integration with lab information management systems (LIMS), chromatography data systems (CDS), or other critical lab instruments & software is essential. ELNs are supposed to simplify and improve processes so it's important that these integrations are able to connect your entire lab and support automated workflows.
- Scalability and flexibility: An ELN has to be scalable enough to handle large datasets and flexible enough to adapt to evolving research methodologies and projects.
Labs need to be able to move fast and deal with rapid shifts. Consider rapid shifts in research focus for many labs during the development of COVID-19 vaccines and treatments. ELN's need to be flexible enough to support your unique research processes and scalable enough to grow with you.
Define Success Metrics
It's important to know what your aims are with the adoption of an ELN. Is it to improve traceability? Have better access to data and lab results? Reduce compliance issues? Or is it just to digitise and modernise your lab? Whatever it is, you should define what results you hope to achieve.
Here are some factors you might want to consider:
- Reduce data retrieval times
- Reduce regulatory compliance issues
- Increase accessibility to data, documents, and results
It's also useful to try and benchmark the success of your ELN to assess how effective an asset it is and whether you are maximising its value. When benchmarking, try to make this as quantifiable as you can.
Organise your existing notebooks and data
Begin by undergoing a detailed inventory of all data types documented in traditional paper notebooks. In pharmaceutical research, this may include not only experimental data and results but also observations, protocols, batch records, and quality control (QC) checks.
This step is essential in terms of making sure everything is traceable as every data point could be necessary for regulatory scrutiny or patent filings.
Expert Insight: Use a systematic approach for cataloguing data. You should use a standardised taxonomy that is specific to your lab's specific research areas and regulatory compliance. For example, you could categorise data based on project codes, study phases (e.g., preclinical, Phase I-III), and data types (e.g., analytical, biological, chemical).
This step will not only help you evaluate your needs when selecting an ELN, but it will also make it much easier when actually migrating everything across to your new system.
Here are some more quick tips on naming existing data & documents:
- Notebook titles: Combine user initials, year, and project name (e.g., JDOE-2024-VaccineDev).
- Page entries: Format with experiment number, name, user initials, and date (e.g., 02-AntigenTest-JDOE-20240321).
- File names: Clearly state the experiment and data specifics (e.g., VaccineTrial1-Data.xlsx).
Create an implementation plan
When making the transition from laboratory notebook to ELN, it's a good idea to come up with a clear plan of how this will be done and how long it will take. Your ELN system provider should technically be doing this for you and making the process as easy as possible. But failing that, it's helpful to know what you need to plan for.
Timeline and milestones
Develop a clear timeline that spells out the key milestones in the ELN implementation process. For a biotech company, these milestones might include regulatory compliance checks, integration testing with existing lab instruments and systems, and pilot test phases that are coordinated with existing project cycles or even regulatory submission deadlines.
You should include buffer periods for unexpected delays, especially around phases that involve regulatory compliance verification or complex data migrations.
Try to be realistic about how long this transition will take. Account for data & document transfers, setting-up your new platform environment, and training your teams properly. If you have a particularly large organisation or complex processes, it can take upwards of 3 months with some vendors.
Regulatory approval gates
Include specific checkpoints for regulatory compliance validation, ensuring that your new ELN meets all necessary guidelines (e.g. FDA 21 CFR Part 11).
Assign roles and responsibilities
These kinds of changes can involve a lot for a company. So it can be helpful form a cross-functional team to oversee the ELN implementation.This team may be made up of employees from R&D, quality assurance, and regulatory affairs
Assign clear roles and responsibilities for all relevant personnel. You should ensure that there is clear ownership over all aspects of the transition.
Going further, it can also be useful to appoint a project manager or an IT specialist to manage system integration and technical issues.
Pilot testing
Choose a small, diverse team to run the pilot test on. It should comprise of researchers, lab technicians, and data analysts who are already engaged in a current, active research project.
This group should represent a cross-section of your lab's operations, including those working on drug discovery, clinical trials, and quality control. This diversity helps ensure that the ELN is tested across a range of research activities and data types common in pharmaceutical and biotech labs.
Collect detailed feedback from all pilot group members, focusing on both the technical aspects of the ELN and its impact on daily research activities. Use this feedback to make informed decisions about broadening the ELN's rollout within the lab and to iterate on training and support structures to ensure wider adoption is successful.
Training
Onboarding involves familiarising your team with an ELN system and training them to a point where they can easily navigate and use the system on a daily basis.
This may involve an initial training of one or two users, usually a team manager, to be a “super” user. This user would ideally be equipped with knowledge of how the platform works and can then disseminate knowledge about the system to the rest of the team.
It's also useful for the “super user” to have additional permission and controls which enables them to oversee and override all activities.
Make sure to take into consideration the timeline for onboarding. Users will need to be trained and be fully competent with the system. The onboarding process may be split into different phases which enables users to navigate different aspects of the platform. The onboarding process should act as a safety net and enables users to make “mistakes” and make any necessary changes to the platform to optimise their workflows.
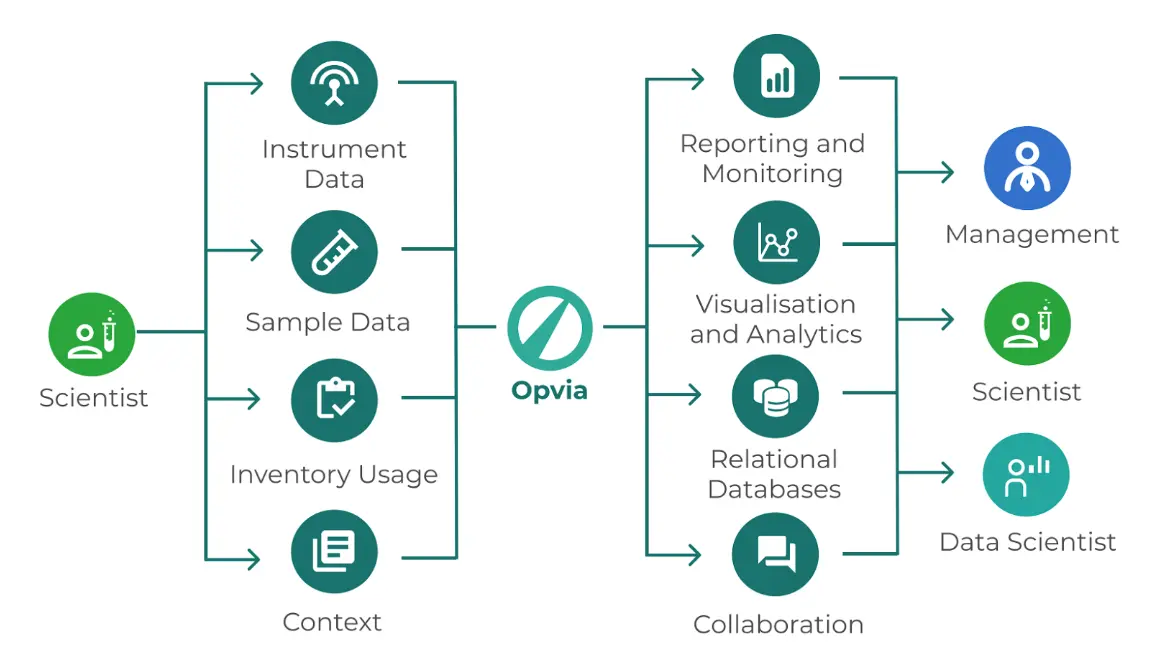
Rely on Your Provider
One of the most valuable resources at your disposal is your ELN provider. Using their expertise and support services will smoothen the transition process and make sure that you're getting the most out of your new system.
Here's how you can get the best guidance from your provider:
- Use support services: Your ELN provider should offer a range of support services, including consultations, technical assistance, and training. Make sure to take full advantage of these offerings not just when adopting your ELN but beyond the implementation phase. Whether it's troubleshooting technical glitches or helping you improve your workflow, the support services provided by your vendor can be invaluable.
- Request customisations: Each lab has unique workflows and preferences. Your ELN provider should offer customisations and build a solution around your needs, not the other way around. Whether it's modifying templates, automating a step in a process, or integrating with different tools, don't hesitate to discuss these needs.
Try to avoid one-size-fits-all templates when selecting an electronic lab notebook. Generic templates, workflows, and systems tend to actually slow you down a lot more than more purpose-built templates. So aim to find a system that doesn't require you to adjust your practices to a stringent framework.

