Good Distribution Practices (GDP) are vital in the pharmaceutical industry to make sure products are handled, stored, and transported safely throughout the supply chain. This guide provides practical insights into GDP compliance, covering key principles, implementation steps, and certification processes. Understanding and adhering to GDP standards is essential for regulatory compliance and maintaining the quality of pharmaceutical products.
What is Good Distribution Practice?
Good Distribution Practice (GDP) is a set of guidelines that make sure medicinal products are stored, transported, and handled properly throughout the supply chain. These practices are vital for keeping medicines safe and effective from the manufacturer to the patient. GDP compliance is important for any business involved in the pharmaceutical supply chain to meet regulatory standards and ensure that the medicines they deliver are safe for use. GDP compliance is part of a set of standards called GxP (‘good practice’).
The Principles GDP Compliance
The European Union (EU) outlines ten key principles of GDP compliance, which cover every aspect of the distribution process. Here’s a simplified breakdown of these principles:
Quality Management System (QMS)
Implement and maintain a quality management system that ensures continuous compliance with GDP standards.
Personnel
Make sure all staff involved in distribution are properly trained and qualified to handle medicinal products safely.
Premises and equipment
Ensure storage and transportation facilities meet standards for temperature control, cleanliness, and security.
Documentation
Keep accurate and complete records of all distribution activities, including handling complaints and product recalls.
Operations
Define clear procedures for receiving, handling, and dispatching medicinal products, focusing on quality control and security.
Complaints, returns, suspected falsified products, and recalls
Set-up procedures for dealing with complaints, managing product returns, and recalling unsafe products.
Outsourced activities
Make sure that any third-party services, like transport companies, follow the same GDP standards as your own operation.
Self-inspections
Regularly inspect your own operations to ensure GDP compliance and correct any issues that arise.
Transportation
Set specific requirements for transporting medicinal products, including maintaining proper temperature and security during transit.
Specific provisions for brokers
Check that brokers, who facilitate transactions without handling the products, also comply with GDP guidelines.
Steps to implement GDP compliance
Implementing Good Distribution Practice (GDP) compliance means that your pharmaceutical products are handled, stored, and transported safely and effectively.
Here’s a practical guide on how to achieve this.
Understand regulatory requirements
First, get a good understanding of the international and regional regulations for GDP compliance:
- United States (US): while GDP isn't standalone in the US, the FDA’s Drug Supply Chain Security Act (DSCSA) outlines requirements for tracking and verifying pharmaceuticals.
- European Union (EU): the EU’s GDP guidelines are pretty comprehensive, covering all aspects of distribution. They emphasise quality management, proper storage, transportation, and maintaining detailed records (as seen in the list above).
- World Health Organisation (WHO): the WHO also offers global guidelines, adopted by many countries, to ensure the safe distribution of medicinal products.

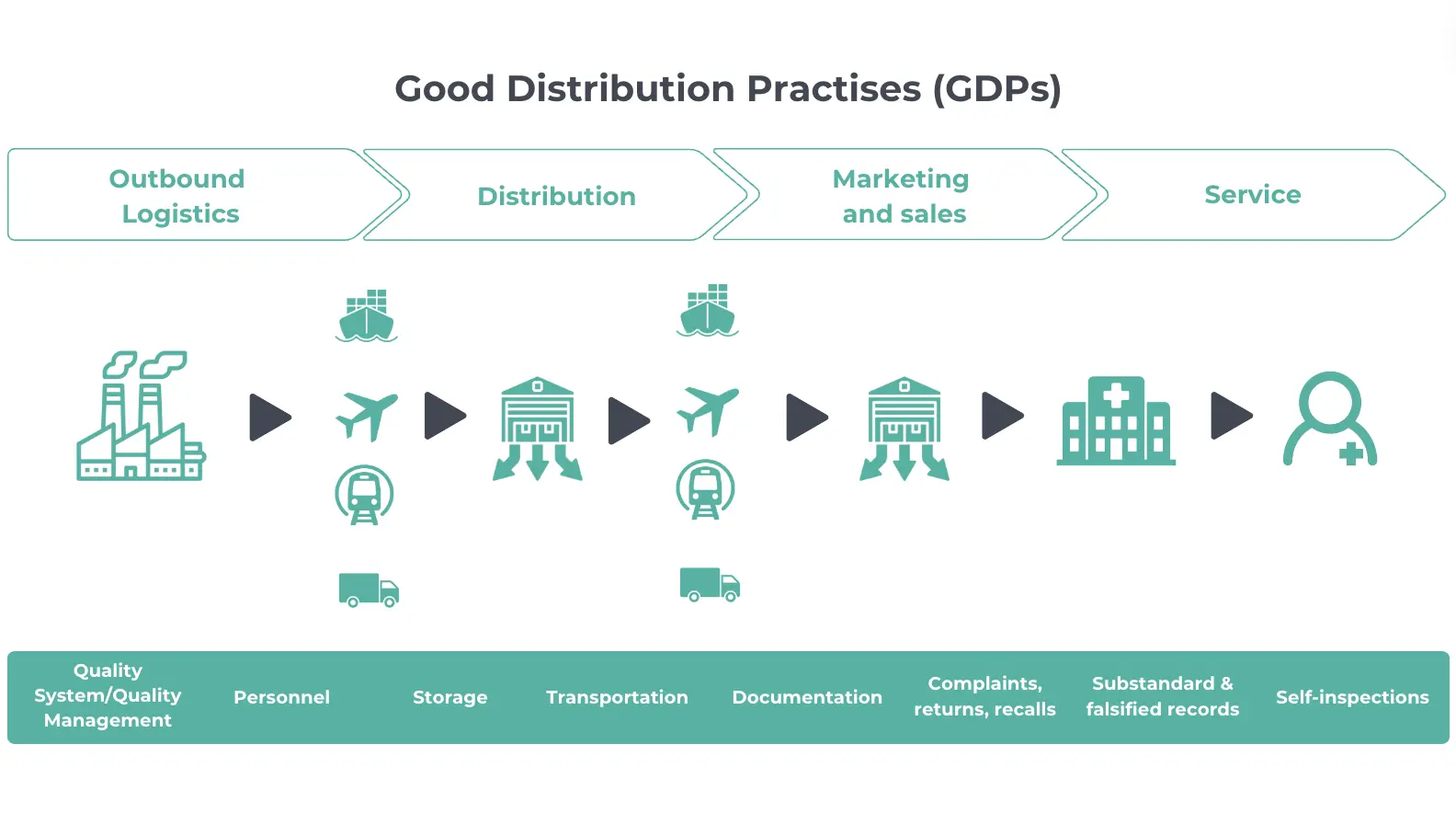
Map your supply chain
Next, map your supply chain to find and mitigate against risks:
- Document every step: outline every step from manufacturing to delivery, making sure to include all processes, facilities, and transport methods.
- Identify risks: look for potential risks at each step, such as temperature fluctuations, contamination, or security issues.
- Implement controls: use tools like temperature monitors, secure packaging, and enhanced security measures to address identified risks.
Establishing a Quality Management System (QMS)
A Quality Management System (QMS) is key for GDP compliance. To achieve this, it’s important to follow these steps:
- Set quality policies and objectives: clearly define your company’s commitment to quality and set specific GDP-related objectives.
- Document procedures: develop Standard Operating Procedures (SOPs) for all critical processes, including storage, transportation, handling, and record-keeping.
- Train your team: ensure all staff involved in distribution understand GDP requirements and your specific procedures. Regular training updates are crucial.
- Monitor and review: Implement systems to monitor compliance with GDP standards and regularly review processes to identify areas for improvement.
- Conduct self-inspections: Perform regular internal audits to check for compliance with GDP guidelines and quickly address any issues found.
Building a Good Distribution Practice Team
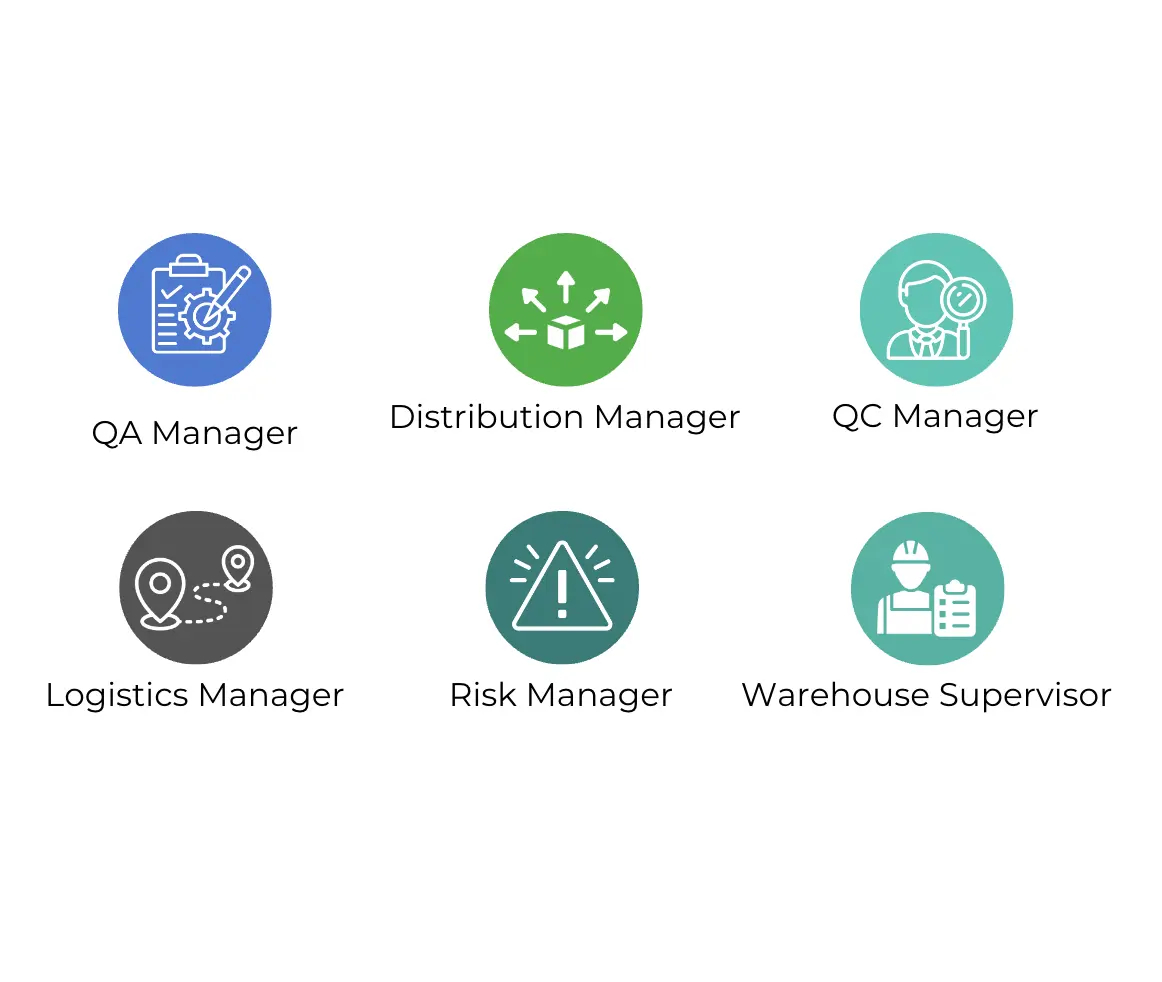
To ensure GDP compliance, you'll need a team with clearly defined roles:
- Quality Assurance (QA) Manager: Oversees quality control, documentation, and compliance monitoring.
- Logistics & Distribution Manager: Handles daily logistics and distribution operations, including storage and transportation.
- Risk Management Specialist: Spots and manages potential supply chain risks, both at the start and periodically.
- Warehouse Supervisors: Ensure products are stored and handled correctly on the ground.
- Quality Control Inspectors: Check warehouses, trucks, and other supply chain parts to make sure good distribution practices are being followed.
A cross-functional approach is key, especially during supply chain mapping and risk assessment. Get input from different parts of the business to catch and handle all risks.
Training and good internal communication are essential to building a team that values and priorities compliance with good distribution practices. Encourage team members to identify and address compliance issues proactively, and reward them for their efforts.
GDP certification process
Certification in the EU
The EU certification process begins with an initial assessment where you contact a competent authority in your country, such as the MHRA in the UK or ANSM in France, to initiate the certification process. Preparation is key; make sure your operations fully comply with EU GDP guidelines, which include having:
- Proper storage facilities
- Trained personnel
- Accurate documentation.
Next, the authority will conduct an inspection of your facilities and processes. This inspection will evaluate your compliance with GDP standards, including how you store, handle, and transport medicinal products. If the inspection is satisfactory, the competent authority will issue a GDP certificate within 90 days, confirming that your operations meet GDP requirements.
Certification in the USA
The approach to GDP certification is different in the US. Instead, compliance is guided by the FDA’s oversight and the Drug Supply Chain Security Act (DSCSA).
So start by familiarising yourself with the DSCSA, which mandates product tracing, verification, and serialisation to ensure the integrity of the supply chain. Again it’s crucial to implement a robust QMS as well as document all processes related to:
- Storage
- Handling
- Transportation
Likewise, you need to make sure that your distribution practices comply with FDA guidelines on proper storage conditions, temperature control, documentation, and handling procedures.
Differences between GMP and GDP
GMP and GDP focus on different stages of the pharmaceutical lifecycle.
GMP (Good Manufacturing Practice)
GMP focuses on ensuring consistent production and control of products according to quality standards. It covers raw materials, facilities, equipment, and staff hygiene. The objective of good manufacturing practice is to minimise risks in the manufacturing process to make sure the final product is safe and effective.
GDP (Good Distribution Practice)
Meanwhile, GDP focuses on the proper storage, transportation, and handling of products to maintain their quality throughout the entire supply chain. Good distribution practice is intended to maintain a product’s integrity from the manufacturer until it reaches the end-user.
In simpler terms, GMP makes sure the product is safe and effective when it leaves the factory, and GDP ensures it stays that way until it gets to the patient.
Compliance of third-party service providers
Making sure your third-party service providers stick to GDP standards is key to keeping pharmaceutical products top-notch.
Start by doing a thorough check of their quality management systems, facilities, and compliance history. Clearly state your GDP compliance expectations in contracts. Regularly audit these providers with on-site inspections and review their documentation. If relevant, you can provide them with training and resources to help meet GDP standards, and keep communication open. Be sure to continuously monitor their performance and address any issues quickly.
Frequently Asked Questions (FAQ)
What is GDP and GMP compliance?
Good Manufacturing Practice (GMP) compliance is about ensuring that medicines are made safely and consistently. It covers everything from the quality of raw materials to the cleanliness of the manufacturing facilities and the training of staff.
Good Distribution Practice (GDP) compliance ensures that medicines are stored, transported, and handled properly after they are made. This ensures that they remain safe and effective until they reach the patient.
In short, GMP focuses on making the medicines, while GDP focuses on keeping them safe during distribution.
What does GDP mean in medical terms?
In medical terms, Good Distribution Practice (GDP) refers to the guidelines that ensure medicines are distributed safely. This includes proper storage, transportation, and handling to keep the medicines effective and safe for patients.
What is pharmaceutical GDP compliance?
Pharma GDP compliance means following specific rules to make sure that medicines are stored and transported correctly. This helps prevent contamination and ensures that medicines stay safe and effective from the factory to the pharmacy.
What is the principle of good distribution practice?
The main principle of Good Distribution Practice (GDP) is to make sure that medicines are stored and transported under the right conditions. This includes maintaining proper temperatures, preventing contamination, and keeping detailed records. The goal is to ensure that medicines are safe and effective when they reach the patient.
What is good distribution practice in quality assurance?
In quality assurance, Good Distribution Practice (GDP) means following guidelines to keep medicines safe during storage and transportation. This includes having a quality management system, training staff, conducting regular audits, and maintaining accurate documentation. GDP helps ensure that the quality of medicines is not compromised during distribution.
What is GDP certified?
A company that is GDP certified has been officially recognised for following GDP guidelines. This certification is given after an inspection by authorities like the MHRA in the UK or the FDA in the US. It means the company’s distribution processes meet all the necessary standards to keep medicines safe and effective throughout the supply chain.

