Manufacturers looking to adopt electronic batch records (EBR systems) often find decision-making difficult. Pinpointing exactly what features will best serve their organisation isn't always easy.
Choosing the system that will drive your entire manufacturing process is not a light decision. These systems can come with high upfront costs, and investing months on implementing a system and training up teams—only to discover it falls short—can be a massive waste of time and money. On top of this, a bad system can grind operations to a halt, lead to non-compliance, or even invoke lawsuits.
There are some horror stories about companies that have made the leap to a new enterprise software solution. Invacare, a medical device manufacturer, had major setbacks during a product update in 2021 when they moved over to a new SAP ERP system. Likewise, MillerCoors aimed to consolidate its finances onto a single ERP system (2014) but faced a long lawsuit as a result of critical defects and poor planning in the project managed by HCL Technologies.
This article is intended to be a handy EBR system comparison guide to help you decide on your EBR. We've carefully evaluated the top 5 EBR systems, ranking them based on crucial criteria like compliance, scalability, and integration capabilities.
What is an EBR?
An Electronic Batch Record (EBR) workflow is a digital process which involves capturing and managing data and documentation during the manufacturing of goods. This kind of advanced workflow is essential in regulated industries with high levels of quality control and compliance, like pharmaceuticals, biotech and the food & beverages.
If you're confused by any of this, we've written other articles about EBRs that you might be interested in:
What makes a great EBR system?
Choosing the right Electronic Batch Record (EBR) system is essential for manufacturers that want to ensure compliance and be as efficient as possible. The best EBR systems stand out by not just meeting this basic criteria but also by being incredibly intuitive to use, customisable and enabling you to have visibility over all your processes.
Here is the key criteria when comparing EBR systems:
Compliance
Clearly, an EBR system needs to be compliant with relevant standards. Otherwise what's the point? Standards like FDA 21 CFR Part 11 are non-negotiable. The chosen system should include features such as audit trails and electronic signatures.
Integration
EBR systems need to be able to integrate seamlessly with other systems like enterprise resource planning (ERP), quality management systems (QMS), and manufacturing execution systems (MES). Systems that offer API access and support standard data exchange formats will make your life a lot easier by accommodating your existing software templates.
Scalability
The ability for an EBR system to scale is pretty crucial. As a business grows, the system should be able to handle increased production without affecting performance. Equally, the provider should be able to accommodate other tools and features in the product life cycle like quality management, R&D, or other manufacturing templates. Try to look for systems that offer modular upgrades, or that can easily expand in both capacity and in functionality offered.
User experience (UX)
A user-friendly interface is important, especially if dealing with complex systems processes. Intuitive navigation, clear display, and easy access to different features go along way in terms of reducing training time and errors. You should also look for mobile/ tablet compatible interfaces that can be used in different circumstances.
Customisability
No two manufacturing processes are the same. An EBR system should be customisable and meet the specific needs of an organisation's workflow. Businesses must be able to customise workflows, reports, and user permissions without needing to code anything or rely heavily on customer support.
Support & maintenance
Providers should offer support plans that include timely updates, bug fixes, and lots of user support. Your team should feel in good hands, with a support team that has extensive technical knowledge not just of the system but of manufacturing processes more generally.
Likewise, the vendor should be continuously improving their solution with regular updates and improvements that make life easier — the product shouldn't feel stagnant or outdated.
Reporting and analytics
Being able to generate reports and perform detailed analytics is another hallmark of a a great EBR system. This includes real-time data visualisation and the ability to track performance metrics. Nowadays this kind of thing is standard for most systems. So a provider should go above and beyond basic visualisations and allow you to really build whatever you aim to track.
EBR system comparison
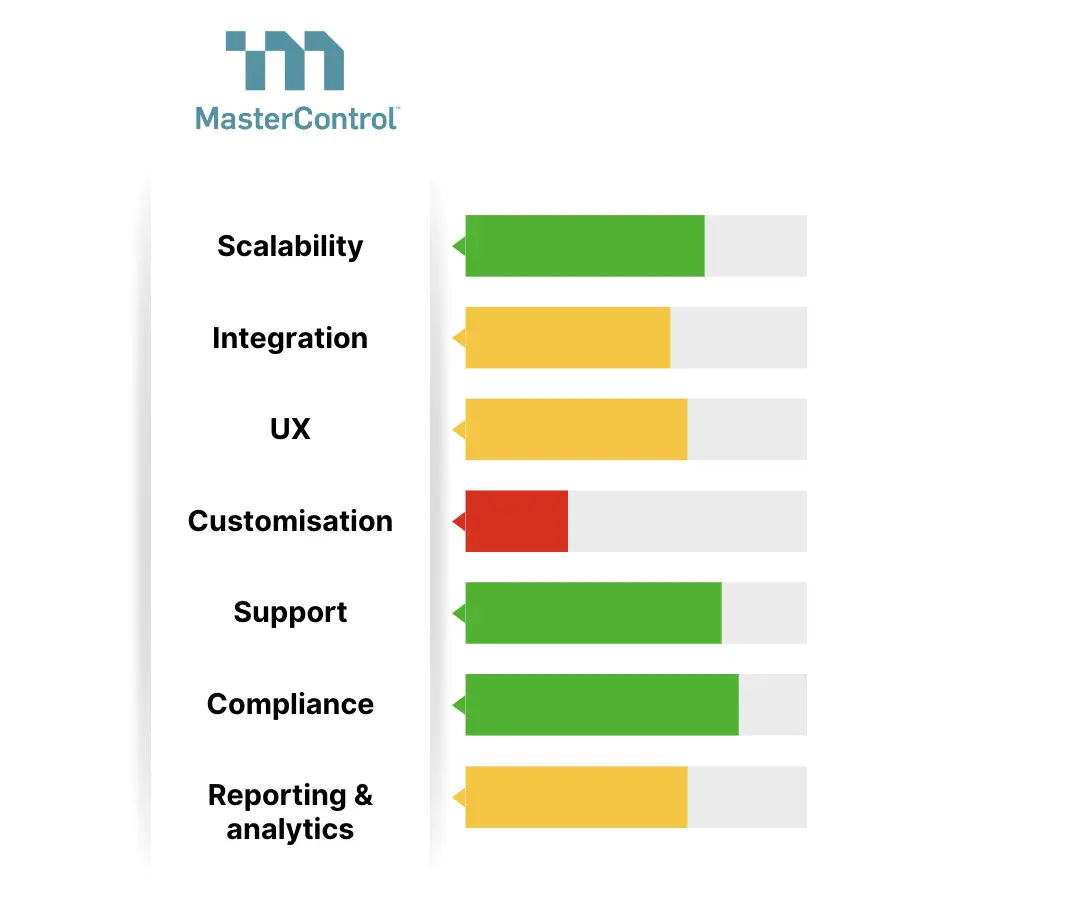
1. MasterControl
MasterControl specialises in templates for pharmaceutical and other regulated industries, offering templates for manufacturing and quality control. One of the products they offer is MasterControl EBR.
Key features:
- Simplifies compliance with audit-ready EBRs, accessible from anywhere
- Integrates with other enterprise systems
- Connects SOPs, training, specifications, and quality events for traceability
Technical specifications:
- Complies with FDA cGMP.
- Integrates with ERP systems for increased data accuracy
- Mobile-friendly dashboard for real-time data access
Pros:
- Reduces manual error
- Better data integrity
- Real-time insights.
Cons:
- Complex to use
- Costly for smaller setups
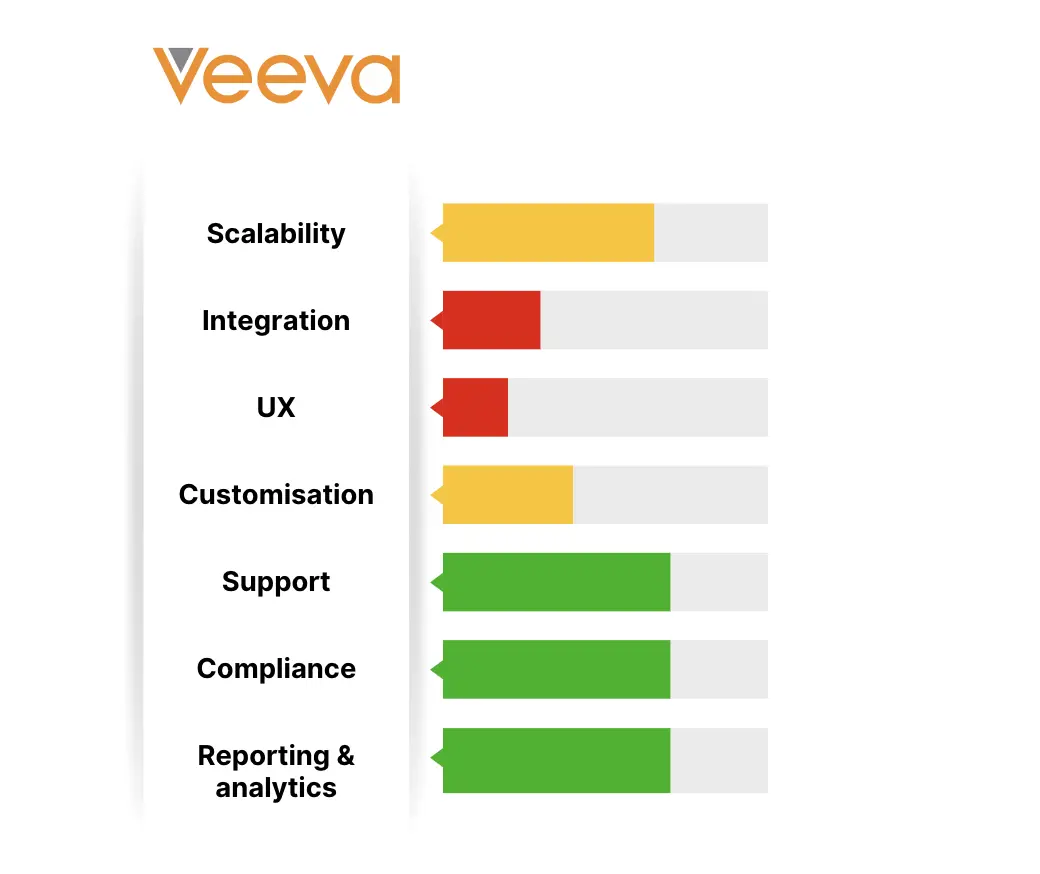
2. Veeva Systems
Veeva Systems offers a Batch Release solution, a platform designed for enterprises in industries including pharmaceuticals, biotechnology, and CDMOs. Introduced in 2023, it automates the aggregation, review, and traceability of batch-related data and content.
Key features:
- End-to-end solution for faster and more confident GMP release and market shipment decisions
- Integrates data and content from QMS, LIMS, ERP, and Vault RIM, for easier collaboration with external partners.
- Streamlines batch release processes
Technical specifications:
- Requires QMS and is typically integrated with Vault QualityDocs and LIMS
- Designed to work best when integrated with Veeva's QMS, QualityDocs, LIMS but it can also interface with third-party templates.
Pros:
- Majes complex processes easier to manage
- Compliance with GMP
Cons:
- High dependency on other Veeva systems, which may limit flexibility for those using more third party systems.
3. Ideagen
Ideagen provides information management software that helps companies with compliance, regulatory, and risk management, particularly in highly regulated industries like aviation, financial services, and life sciences.
Key features:
- Audit management
- Document control
- Process mapping tools.
Technical specifications:
- Can integrate with existing IT infrastructure
- Scalable enough to meet the needs of large enterprises.
Pros:
- Strong emphasis on compliance and risk management
- Good customer service
- Highly adaptable software which can be used for many different industrie
Cons:
- The user interface requires a steep learning curve
- Initial setup can be resource-intensive, with lots of upfront investment and training

