Table of contents
- What is Change Control in the Pharmaceutical Industry?
- What are the change control requirements in the pharmaceutical industry?
- Why Change Control is Crucial in Pharma
- Key Elements of an Effective Change Control Process
- Examples of Change Control in Pharma
- Challenges in Change Control
- Best Practices for Managing Change Control
- What is the Difference Between CAPA and Change Control?
- Frequently Asked Questions (FAQs)
Change control in the pharmaceutical industry is the process of managing changes to processes, systems, or products to ensure they don't compromise quality or compliance. It involves documenting, evaluating, and approving changes before they are implemented. This article will explain what change control is, why it’s crucial for Good Manufacturing Practice (GMP), and outline the key steps in an effective change control process. You'll also learn about common challenges, best practices, and the difference between CAPA (Corrective and Preventive Action) and change control. By the end, you’ll understand how change control helps keep pharmaceutical products safe and effective.
What is Change Control in the Pharmaceutical Industry?
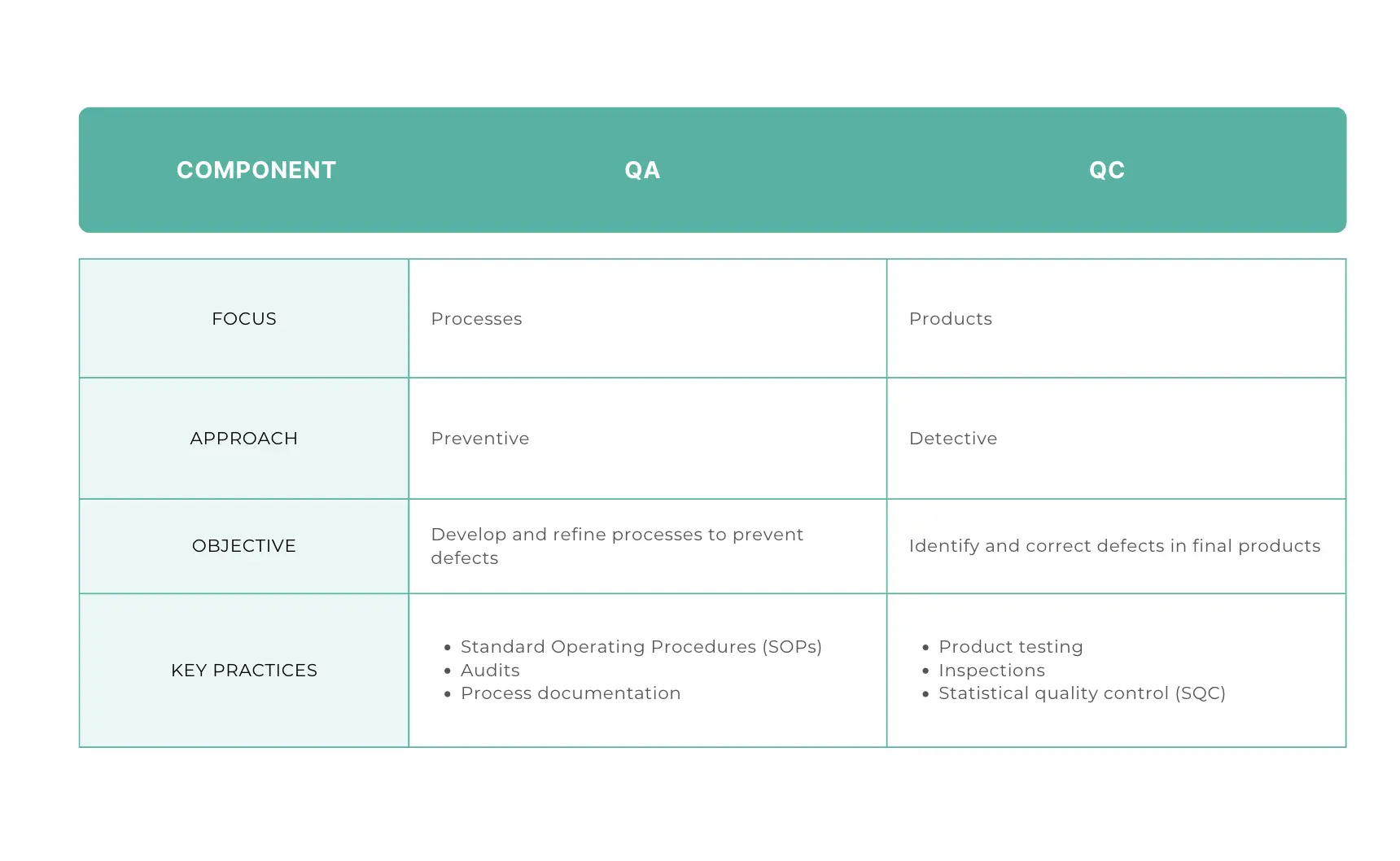
To start, we have to introduce quality assurance and quality control: these are crucial aspects of the pharmaceutical industry, ensuring that products are safe, effective, and meet regulatory standards. ‘Quality assurance’ refers to the systematic processes that provide confidence in a product's quality (for example audits, risk management and training), while ‘quality control’ involves the operational activities used to meet quality requirements (commonly through testing and inspections).
Change control is an integral part of quality assurance - it’s the formal process by which organisations manage modifications to standard processes, systems and products, to ensure that any proposed changes do not negatively impact the company’s ‘validated state’. This is a state where processes, practices, and systems of the company are documented (and verified) to be in compliance with regulatory guidelines.
A strong change control process enables traceability through an organisation, such that the impact of each change on both product quality and regulatory compliance is fully (a) understood and (b) controlled. Industry wide, these processes preserve the safety and integrity of pharmaceutical operations, ensuring alignment with GxP guidelines (including Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Clinical Practice (GCP).
What are the change control requirements in the pharmaceutical industry?
Change control in the pharmaceutical industry is all about making sure any changes made during the manufacturing process don’t compromise the quality and safety of the products. The requirements can vary depending on where the company operates, but here are the most common guidelines explained.
EudraLex Volume 4 GMP
If you’re manufacturing medicines in the EU, EudraLex Volume 4 GMP is your go-to guide. It outlines how pharma companies need to have written procedures for managing any changes in your processes (Chapter 4, Section 4.29).
If you’re changing how you make the product, what equipment you use, or the materials involved, you have to validate those changes to ensure they won’t mess with the product’s quality (Chapter 5, Section 5.25).
Annex 15, Section 11, also highlights using quality risk management to plan and assess these changes, making sure you’ve considered any potential impacts on quality.
EU 1252/2014
This European regulation is for companies dealing with active substances in medicines. Article 14 of EU 1252/2014 requires you to assess how any changes to your manufacturing process might affect the quality of the active substance before you go ahead with those changes.
FDA 21 CFR Part 211
In the US, the FDA’s 21 CFR Part 211 sets out the rules for Good Manufacturing Practice (cGMP) for finished pharmaceutical products. Here are a few key points:
21 CFR 211.22: Your Quality Control Unit needs to approve or reject any changes. They should have all the resources and facilities they need to do this job properly.
21 CFR 211.100: You need written procedures for production and process control, including how to handle changes. These procedures must be created by authorized staff and approved by the Quality Control Unit. If there’s a deviation from these procedures, it needs to be documented and justified.
21 CFR 211.160: In a lab setting, you need written procedures for various processes, including handling changes.
ICH Q10
ICH Q10 provides a model for an effective pharmaceutical quality management system. It says your change control process should give you high confidence that any changes won’t lead to unexpected problems (Section 3.2.3). It also suggests using quality risk management to carefully evaluate any proposed changes.

Why Change Control is Crucial in Pharma
Regulatory requirements
Following regulatory requirements is crucial - regulatory bodies like the FDA (Food and Drug Administration) in the United States, and the EMA (European Medicines Agency) in Europe, impose strict guidelines on pharmaceutical companies. These guidelines (known as ‘GxP’) instruct that any proposed changes that could impact the quality of a product, or patient safety, must be thoroughly evaluated, documented, and approved prior to being implemented. Change control responds to this mandate, acting to protect public health and minimise an organisation’s risk of non-compliance.
Consequences of poor change control practices
Poor change control practices can result in company operations that aren’t aligned with GxP guidance. If perpetuated, this can lead to non-compliance with regulatory requirements, resulting in product recalls, vast financial penalties, and reputational damage. Crucially, a lack of change control can risk harm to patients - errors during the manufacturing process of a medical device, for example, could lead to defective products otherwise intended for life-changing applications.
Key Elements of an Effective Change Control Process
An effective change control process in the pharmaceutical industry follows several key steps.
It all starts with initiation, where a ‘change request’ is created by spotting the need for a change. This request should clearly describe what the change is and why it's needed. Then, during the evaluation phase, the proposed change is carefully looked at to see how it might affect product quality, safety, and compliance. This often involves risk assessments, feasibility studies, and talking with the right stakeholders.
After the evaluation, the change request and findings are submitted for approval. This usually involves a Change Control Board (CCB) made up of quality assurance, regulatory affairs, and other important team members. Once the change is approved, it's time for implementation. This phase involves putting the change into action according to a plan, which might include updating Standard Operating Procedures (SOPs), training staff, and adjusting equipment.
Once a change is implemented, it goes through a verification stage to make sure everything was done correctly and the change achieves the desired results without causing any issues. Throughout the entire process, it's important to keep detailed records, including the initial change request and all the steps taken during evaluation, approval, implementation, and verification. Lastly, regular reviews are done to check how well the change control process is working and to find any areas that could be improved.
Examples of Change Control in Pharma
Changes can occur in various stages of the pharmaceutical product lifecycle. For instance, formulation changes need to undergo rigorous testing and validation to confirm the new formulation is both safe and effective. Process changes, like altering the temperature or mixing time in manufacturing, need thorough evaluation to prevent any negative impact on product quality. When introducing new equipment or upgrading existing machinery, it's important to validate that it operates correctly and does not introduce contamination or errors.
Packaging changes should be assessed for their impact on product stability and protection during transportation and storage. Similarly, any revisions to analytical methods used for product testing need validation to ensure the new methods provide accurate and reliable results. Adapting to new or updated regulatory requirements involves reviewing and updating procedures to maintain compliance.
Challenges in Change Control
Implementing change control is not without its challenges: the complexity of regulatory requirements, which may vary significantly between different regions, can be time consuming to align with. Resistance to change within an organisation, perhaps due to disruption of established workflows and migration from legacy systems, can also pose a hurdle. Finally, managing the documentation and approval process associated with change control can be resource-intensive.
It is common for companies to look to electronic templates to service some of these requirements.
Best Practices for Managing Change Control
First, set up a formal change control system. This means having a team from different departments review proposed changes to make sure everything stays validated and in good working order. Use detailed forms to document each change request, including why it's needed and what it will impact, to keep things transparent and easy to track.
Next, perform thorough impact assessments. This step helps you understand how the change might affect product quality, safety, and compliance. Conduct risk assessments and feasibility studies to spot and manage any potential issues. The Change Control Board (CCB)—including members from quality assurance, regulatory affairs, and other key departments—reviews and approves changes to ensure they meet all standards and policies.
Once a change is approved, implement it according to a well-thought-out plan. This might involve updating Standard Operating Procedures (SOPs), retraining staff, and making equipment modifications. Careful planning ensures everything goes smoothly. After implementation, verify and validate the change to make sure it achieves the desired outcomes without causing new problems. This involves testing and monitoring to confirm everything is working as expected.
Keep detailed documentation at every step. This includes records of the change request, impact assessments, approvals, implementation details, and verification results. Good documentation is crucial for traceability and regulatory audits. Regularly review your change control process to see how well it’s working and find areas for improvement, helping your system stay effective and up-to-date.
What is the Difference Between CAPA and Change Control?
CAPA (Corrective and Preventive Action) and change control are both crucial elements of a pharmaceutical company's quality management system, but they serve different purposes.
CAPA focuses on identifying and addressing root causes of non-conformances or deviations to prevent their recurrence, involving “corrective actions” to fix existing problems and “preventive actions” to avoid future issues.
Change control, on the other hand, is a proactive process - it manages planned changes to processes, products or systems.
Frequently Asked Questions (FAQs)
What is Change Control in Pharma?
Change control in the pharmaceutical industry is a way to manage any modifications to processes, systems, or products to make sure they don’t mess up the quality or compliance. It involves documenting, evaluating, and approving changes before they happen to ensure everything stays on track and meets regulatory standards like GMP (Good Manufacturing Practice).
Why is Change Control Important to GMP?
Change control is key for maintaining Good Manufacturing Practice (GMP) because it ensures that any changes affecting product quality, safety, and compliance are properly checked and approved. This helps prevent issues like product recalls, fines, or harm to patients, keeping the company compliant with regulations set by bodies like the FDA and EMA.
What is the Difference Between CAPA and Change Control?
CAPA (Corrective and Preventive Action) and change control are both crucial for quality management but serve different purposes. CAPA is about fixing and preventing problems when things go wrong. It looks at what caused the issue and how to prevent it from happening again. Change control, on the other hand, deals with planned changes, ensuring these changes don’t negatively impact the company’s validated state or product quality.
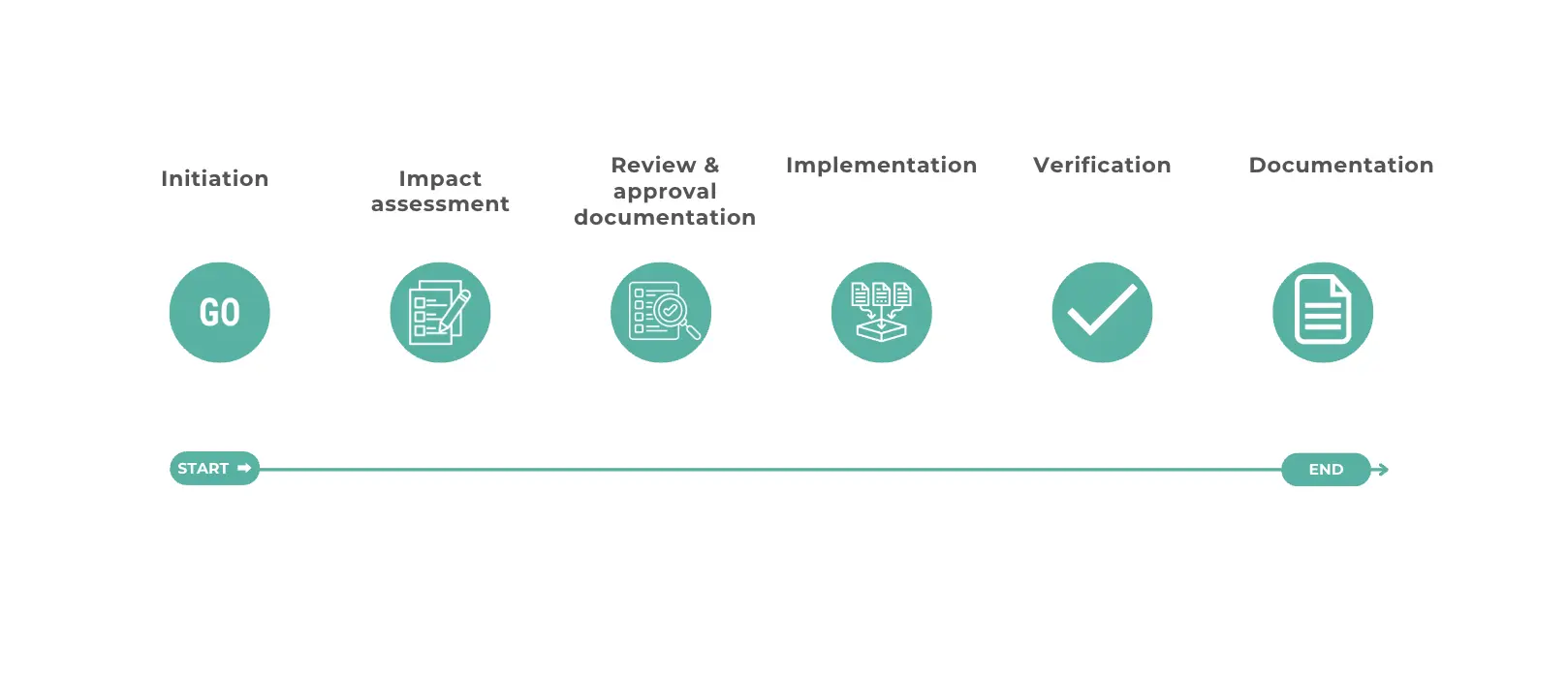
What are the Six Steps in the Change Control Process?
The six steps in the change control process are:
- Initiation
- Impact Assessment
- Review and Approval
- Implementation
- Verification
- Documentation
What is QMS Change Control?
QMS (Quality Management System) change control is about managing changes within the quality system itself, like updating electronic QMS or altering standard procedures. It ensures these changes are carefully planned, evaluated, and documented to keep the quality management system effective and compliant with regulations.

