There are two essential regulatory frameworks that help achieve high levels of compliance and quality in the pharmaceutical industry. These are Current Good Manufacturing Practices (cGMP) and Good Laboratory Practices (GLP). While both are essential for compliance, they have quite different purposes and are applied at different stages of the product lifecycle. This article explains the key differences between cGMP and GLP, and their roles in upholding quality in the pharmaceuticals sector.
What is cGMP (Current Good Manufacturing Practices)?
Definition
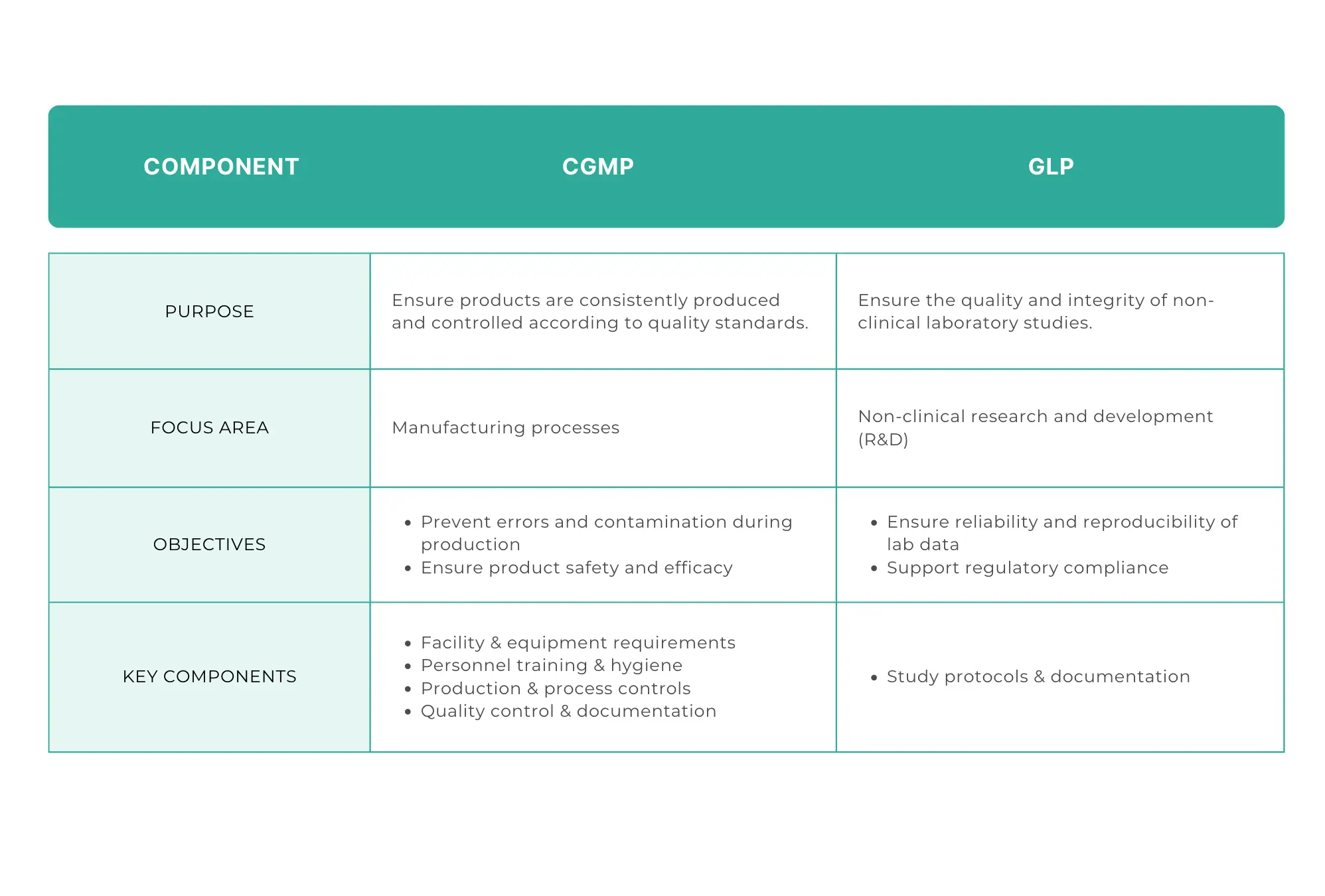
Current Good Manufacturing Practices (cGMP) are a set of regulations enforced by the FDA to make sure that pharmaceutical products are produced consistently and to high quality standards.
cGMP pharmaceutical manufacturing from end-to-end. From raw material procurement all the way to final product distribution. The aim of cGMP is to prevent errors, and to ensure the safety and quality of pharma products.
Key Components of cGMP
Facility & equipment requirements: cGMP specifies that manufacturing facilities should have a clean and controlled environment to avoid contamination. This means that there should be strict requirements for the layout and maintenance of facilities. The equipment used in manufacturing should be properly maintained and regularly calibrated.
Personnel training & hygiene: employees should receive training in cGMP standards and processes. Likewise, high levels of personal hygiene are important to avoid letting contaminants into the manufacturing site.
Production & process controls: standardised production processes are crucial for product quality. cGMP states that there should be detailed documentation of every step in the manufacturing process, from the receipt of raw materials to the final packaging of products. This kind of really thorough documentation again means that there's consistency and traceability. It also means that you can easily identify and correct any deviations from standard procedures.
Quality control & documentation: documentation is another essential part of cGMP. This includes detailed records of production batches, quality control tests, and regular audits. Documentation helps make sure that all products meet the required standards before they reach the market. It also offers clear evidence when it comes time for regulatory inspections.
What is GLP (Good Laboratory Practices)?
Definition
Good Laboratory Practices (GLP) are a set of principles intended to ensure the quality of non-clinical laboratory studies. These practices are essential for creating reliable and reproducible data, especially in the context of R&D for pharmaceuticals. GLP regulations are applied to make sure that lab data of products is credible and meets regulatory standards.
Key components of GLP
Study protocols & documentation: GLP requires detailed study protocols and documentation for every procedure and result. Study protocols should be clearly defined and followed to a T. Documentation is important for checking that studies are conducted as they were planned and for reproducing results, if needed.
Personnel qualifications & training: As with cGMP, staff in the lab must be well-qualified and trained to maintain high standards of performance and data integrity. Every member of staff should also have specific roles and responsibilities.
Facilities & equipment: lab facilities and equipment need to be properly maintained and calibrated. Likewise, proper maintenance of lab sites and equipment prevent errors and ensures the reliability of test results.
Quality assurance programs: Quality assurance programs involve regular reviews and audits of laboratory practices to follow GLP standards. These internal and external audits help find any deviations from protocols and offer opportunities to make corrective actions.
Key Differences Between cGMP and GLP
Manufacturing vs. R&D
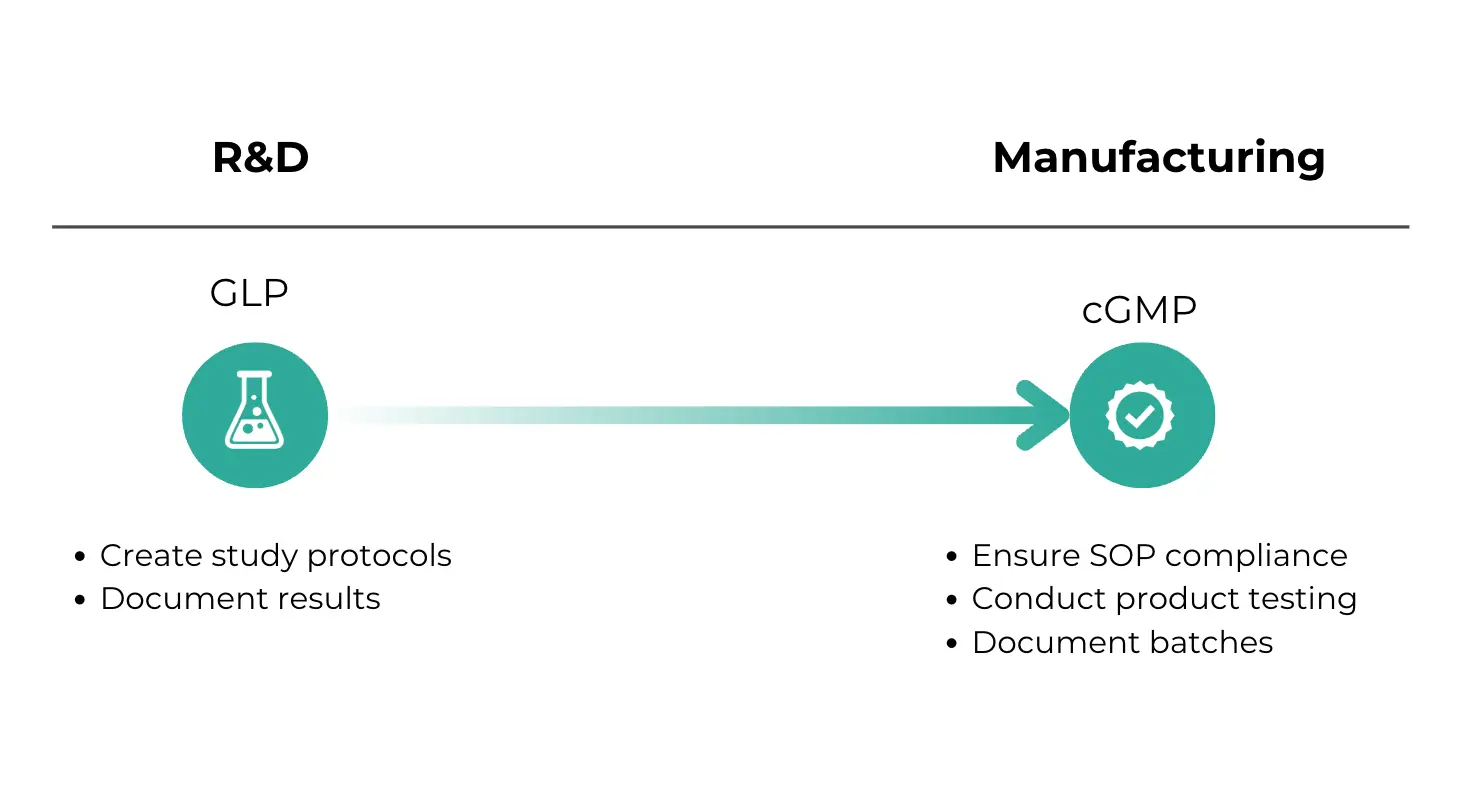
The main distinction between cGMP and GLP is in their focus area.
cGMP is focused on manufacturing processes and making sure that a product is safe. It covers all the stages of production, like raw material procurement, manufacturing, packaging, and distribution. The main goal of cGMP is to prevent errors during production and check that every product batch meets required specifications.
On the other hand, GLP focuses on the integrity and quality of laboratory data generated during non-clinical research. It's concerned with the processes within the laboratory setting, like study protocols, data collection, and reporting. The aim of GLP is to make sure that the data produced in the lab is reliable and reproducible, so that R&D teams can make validated decisions on whether to further develop a product and whether it will pass regulatory approval.
Preventive vs. Data integrity
Another difference is the approach each standard takes towards quality.
cGMP is preventive. It aims to establish and maintain manufacturing protocols that prevent defects from ever occurring in the first place. This involves lots of process controls, equipment maintenance, and employee training.
In contrast, GLP focuses on data integrity. It makes sure that all laboratory studies are done following strict protocols, and that the data generated is reliable. The emphasis here is on verifying data, rather than preventing errors.
Techniques & Tools
Both cGMP and GLP have specific tools, processes and documents types to achieve their respective goals.
cGMP Techniques and Tools:
- Standard Operating Procedures (SOPs)
- Production Audits
- Process Validation ie. testing & confirming that processes produce consistent, high-quality products.
- Risk management
GLP Techniques and Tools:
- Study protocols ie. detailed plans that outline the objectives, methodology, and procedures of every study.
- Equipment calibration
- Data recording
- Quality assurance audits
How cGMP and GLP Work Together in the Pharma Industry
In practice, cGMP and GLP are often integrated to cover the most of the lifecycle of a pharmaceutical product. Here's how they work together might work together in an example:
Research Phase (GLP): A pharmaceutical company conducts toxicity and efficacy studies following GLP guidelines. They follow detailed protocols, and all that data is carefully documented and audited.
Manufacturing Phase (cGMP): Once the drug moves into production, cGMP standards help ensure that the manufacturing is controlled and that processes are standardised.
This means that the facilities are regularly cleaned, equipment is properly calibrated, and staff is trained. Documentation of each step in the manufacturing process ensures consistency and traceability. Quality control tests are performed at various stages to verify that the product meets the necessary standards.